Abstract
RNA editing is increasingly recognized as a molecular mechanism regulating RNA activity and recoding proteins. Here we surveyed the global landscape of RNA editing in human brain tissues and identified three unique patterns of A-to-I RNA editing rates during cortical development: stable high, stable low and increasing. RNA secondary structure and the temporal expression of adenosine deaminase acting on RNA (ADAR) contribute to cis- and trans-regulatory mechanisms of these RNA editing patterns, respectively. Interestingly, the increasing pattern was associated with neuronal maturation, correlated with mRNA abundance and potentially influenced miRNA binding energy. Gene ontology analyses implicated the increasing pattern in vesicle or organelle membrane-related genes and glutamate signaling pathways. We also found that the increasing pattern was selectively perturbed in spinal cord injury and glioblastoma. Our findings reveal global and dynamic aspects of RNA editing in brain, providing new insight into epitranscriptional regulation of sequence diversity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
BioProject
Sequence Read Archive
Referenced accessions
Gene Expression Omnibus
References
Savva, Y.A., Rieder, L.E. & Reenan, R.A. The ADAR protein family. Genome Biol. 13, 252 (2012).
Nishikura, K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 79, 321–349 (2010).
Pullirsch, D. & Jantsch, M.F. Proteome diversification by adenosine to inosine RNA editing. RNA Biol. 7, 205–212 (2010).
Rueter, S.M., Dawson, T.R. & Emeson, R.B. Regulation of alternative splicing by RNA editing. Nature 399, 75–80 (1999).
Schoft, V.K., Schopoff, S. & Jantsch, M.F. Regulation of glutamate receptor B pre-mRNA splicing by RNA editing. Nucleic Acids Res. 35, 3723–3732 (2007).
Rosenthal, J.J.C. & Seeburg, P.H. A-to-I RNA editing: effects on proteins key to neural excitability. Neuron 74, 432–439 (2012).
Paul, M.S. & Bass, B.L. Inosine exists in mRNA at tissue-specific levels and is most abundant in brain mRNA. EMBO J. 17, 1120–1127 (1998).
Li, J.B. et al. Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science 324, 1210–1213 (2009).
Ramaswami, G. et al. Identifying RNA editing sites using RNA sequencing data alone. Nat. Methods 10, 128–132 (2013).
Higuchi, M. et al. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature 406, 78–81 (2000).
Li, J.B. & Church, G.M. Deciphering the functions and regulation of brain-enriched A-to-I RNA editing. Nat. Neurosci. 16, 1518–1522 (2013).
Sakurai, M. et al. A biochemical landscape of A-to-I RNA editing in the human brain transcriptome. Genome Res. 24, 522–534 (2014).
Li, Z. et al. Evolutionary and ontogenetic changes in RNA editing in human, chimpanzee, and macaque brains. RNA 19, 1693–1702 (2013).
Blow, M., Futreal, P.A., Wooster, R. & Stratton, M.R. A survey of RNA editing in human brain. Genome Res. 14, 2379–2387 (2004).
Dillman, A.A. et al. mRNA expression, splicing and editing in the embryonic and adult mouse cerebral cortex. Nat. Neurosci. 16, 499–506 (2013).
Athanasiadis, A., Rich, A. & Maas, S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol. 2, e391 (2004).
Kim, D.D.Y. et al. Widespread RNA editing of embedded Alu elements in the human transcriptome. Genome Res. 14, 1719–1725 (2004).
Ramaswami, G. & Li, J.B. RADAR: a rigorously annotated database of A-to-I RNA editing. Nucleic Acids Res. 42, D109–D113 (2014).
Eggington, J.M., Greene, T. & Bass, B.L. Predicting sites of ADAR editing in double-stranded RNA. Nat. Commun. 2, 319 (2011).
Wang, I.X. et al. ADAR regulates RNA editing, transcript stability, and gene expression. Cell Rep. 5, 849–860 (2013).
Sauvageau, M. et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. eLife 2, e01749 (2013).
Fertuzinhos, S. et al. Laminar and temporal expression dynamics of coding and noncoding RNAs in the mouse neocortex. Cell Rep. 6, 938–950 (2014).
Darmanis, S. et al. A survey of human brain transcriptome diversity at the single cell level. Proc. Natl. Acad. Sci. USA 112, 7285–7290 (2015).
van de Leemput, J. et al. CORTECON: a temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron 83, 51–68 (2014).
Hubbard, K.S., Gut, I.M., Lyman, M.E. & McNutt, P.M. Longitudinal RNA sequencing of the deep transcriptome during neurogenesis of cortical glutamatergic neurons from murine ESCs. F1000Res. 2, 35 (2013).
Rybak-Wolf, A. et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell 58, 870–885 (2015).
Luhmann, H.J., Fukuda, A. & Kilb, W. Control of cortical neuronal migration by glutamate and GABA. Front. Cell. Neurosci. 9, 4 (2015).
Kwon, H.-B. & Sabatini, B.L. Glutamate induces de novo growth of functional spines in developing cortex. Nature 474, 100–104 (2011).
Sekine, S., Miura, M. & Chihara, T. Organelles in developing neurons: essential regulators of neuronal morphogenesis and function. Int. J. Dev. Biol. 53, 19–27 (2009).
Slotkin, W. & Nishikura, K. Adenosine-to-inosine RNA editing and human disease. Genome Med. 5, 105 (2013).
Chen, K. et al. RNA-seq characterization of spinal cord injury transcriptome in acute/subacute phases: a resource for understanding the pathology at the systems level. PLoS One 8, e72567 (2013).
Birnbaum, R., Jaffe, A.E., Hyde, T.M., Kleinman, J.E. & Weinberger, D.R. Prenatal expression patterns of genes associated with neuropsychiatric disorders. Am. J. Psychiatry 171, 758–767 (2014).
Ripke, S. et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Daniel, C., Silberberg, G., Behm, M. & Öhman, M. Alu elements shape the primate transcriptome by cis-regulation of RNA editing. Genome Biol. 15, R28 (2014).
Daniel, C., Venø, M.T., Ekdahl, Y., Kjems, J. & Öhman, M. A distant cis acting intronic element induces site-selective RNA editing. Nucleic Acids Res. 40, 9876–9886 (2012).
Garncarz, W., Tariq, A., Handl, C., Pusch, O. & Jantsch, M.F. A high-throughput screen to identify enhancers of ADAR-mediated RNA-editing. RNA Biol. 10, 192–204 (2013).
Jepson, J.E.C. & Reenan, R.A. RNA editing in regulating gene expression in the brain. Biochim. Biophys. Acta 1779, 459–470 (2008).
Horsch, M. et al. Requirement of the RNA-editing enzyme ADAR2 for normal physiology in mice. J. Biol. Chem. 286, 18614–18622 (2011).
Palladino, M.J., Keegan, L.P., O'Connell, M.A. & Reenan, R.A. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell 102, 437–449 (2000).
Jepson, J.E.C. et al. Engineered alterations in RNA editing modulate complex behavior in Drosophila: regulatory diversity of adenosine deaminase acting on RNA (ADAR) targets. J. Biol. Chem. 286, 8325–8337 (2011).
Balik, A., Penn, A.C., Nemoda, Z. & Greger, I.H. Activity-regulated RNA editing in select neuronal subfields in hippocampus. Nucleic Acids Res. 41, 1124–1134 (2013).
Sanjana, N.E., Levanon, E.Y., Hueske, E.A., Ambrose, J.M. & Li, J.B. Activity-dependent A-to-I RNA editing in rat cortical neurons. Genetics 192, 281–287 (2012).
Pinto, Y., Cohen, H.Y. & Levanon, E.Y. Mammalian conserved ADAR targets comprise only a small fragment of the human editosome. Genome Biol. 15, R5 (2014).
Sarkisian, M.R. & Guadiana, S.M. Influences of primary cilia on cortical morphogenesis and neuronal subtype maturation. Neuroscientist 21, 136–151 (2015).
Valente, E.M., Rosti, R.O., Gibbs, E. & Gleeson, J.G. Primary cilia in neurodevelopmental disorders. Nat. Rev. Neurol. 10, 27–36 (2014).
Han, L. et al. The genomic landscape and clinical relevance of A-to-I RNA editing in human cancers. Cancer Cell 28, 515–528 (2015).
La Via, L. et al. Modulation of dendritic AMPA receptor mRNA trafficking by RNA splicing and editing. Nucleic Acids Res. 41, 617–631 (2013).
Zhang, L., Yang, C.-S., Varelas, X. & Monti, S. Altered RNA editing in 3′ UTR perturbs microRNA-mediated regulation of oncogenes and tumor-suppressors. Sci. Rep. 6, 23226 (2016).
Wang, Q. et al. ADAR1 regulates ARHGAP26 gene expression through RNA editing by disrupting miR-30b-3p and miR-573 binding. RNA 19, 1525–1536 (2013).
Jaffe, A.E. et al. Developmental regulation of human cortex transcription and its clinical relevance at single base resolution. Nat. Neurosci. 18, 154–161 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P.T. & Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
DePristo, M.A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011).
Van der Auwera, G.A. et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics 43, 1–33 (2013).
Sherry, S.T. et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 29, 308–311 (2001).
Kleinman, C.L. & Majewski, J. Comment on “Widespread RNA and DNA sequence differences in the human transcriptome”. Science 335, 1302 author reply 1302 (2012).
Lorenz, R. et al. ViennaRNA Package 2.0. Algorithms Mol. Biol. 6, 26 (2011).
Enright, A.J. et al. MicroRNA targets in Drosophila. Genome Biol. 5, R1 (2003).
Griffiths-Jones, S., Saini, H.K., van Dongen, S. & Enright, A.J. miRBase: tools for microRNA genomics. Nucleic Acids Res. 36, D154–D158 (2008).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164–e164 (2010).
Jaffe, A.E. et al. Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex. Nat. Neurosci. 19, 40–47 (2016).
Love, M.I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Adzhubei, I.A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Choy, J.Y.H., Boon, P.L.S., Bertin, N. & Fullwood, M.J. A resource of ribosomal RNA-depleted RNA-Seq data from different normal adult and fetal human tissues. Sci. Data 2, 150063 (2015).
Bernstein, B.E. et al. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Acknowledgements
We are thankful for the vision and generosity of the Lieber and Maltz families who founded the Lieber Institute for Brain Development. We dedicate this work to the memory of Constance Lieber, our inspirational founder and director. We also gratefully acknowledge and thank the families of the donors whose tissues were used in this study. This work was supported by the Lieber Institute for Brain Development, and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A09057171) in Korea. A.K.K.L. thanks the Johns Hopkins Bloomberg School of Public Health start-up fund.
Author information
Authors and Affiliations
Contributions
J.H.S. and D.R.W. designed the project and oversaw all aspects of the studies. T.H. developed the computational pipeline for identifying RNA editing sites and performed the computational analyses as well as exome and targeted DNA sequencing experiments. C.-K.P. provided glioblastoma patient samples, generated RNA-seq on them and assisted the clinical interpretations. A.K.L.L. consulted on the molecular experiments and assisted in the biological interpretation of the computational findings. Y.G. designed the project and oversaw the RNA-seq data generation. T.M.H. and J.E.K. provided brain tissue and demographic data and assisted in the biological interpretation of fetal samples. A.R. helped with sequencing experiments. R.T. performed RNA extractions. T.H. and D.R.W. wrote the manuscript with inputs from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 A-to-I editing sites with increasing patterns across human brain development.
(a) The cumulative proportion of the increasingly edited sites according to the number of samples at which editing sites occur: only 14% of sites were found in 10 samples or less. In other words, 86% of the increasingly-edited sites were found in more than 10 samples. (b) The significant sites determined by ANOVA followed by FDR-correction have increasing editing patterns which are represented by five groups (noted by numbers in a heatmap and regression lines) based on hierarchical clustering. Lines are generated by fitting generalized additive models (GAM) to editing rates in each cluster. Shades indicate 95% confidence interval.
Supplementary Figure 2 Quantification of degree of double-strandedness and the associated distance for an A-to-I editing site.
The degree of double-strandedness for a given A-to-I editing site was calculated from computationally-predicted RNA-structure. First, RNA structure was predicted by RNAfold (rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi) using a pre-mRNA sequence spanning 800 bp upstream and downstream from a given editing site. Second, the number of nucleotides in a double-stranded configuration was counted within a flanking 100 bp region for every position on a pre-mRNA sequence (moving-window search). In the figure, this is depicted by the rolling window of 5 nucleotides for simplicity. Finally, after the site associated with the maximum value within 500 bp upstream and downstream from an editing site was identified, the maximum value was taken as the degree of double-strandedness and the associated distance was calculated.
Supplementary Figure 3 Comparison of the distances associated with the degree of double-strandedness
The groups were compared in terms of the distance associated with the degree of double-strandedness (see methods and Supplementary Fig. 2). There is a significance difference between ‘Group I. low’ and ‘Group II. increasing’ but the significance is weak compared to the degree of double-strandedness in Figure 2c: (ANOVA) N = 163, F(2,160) = 3.917, P-value = 0.0218, (post-hoc two-sided Welch t-test) t(118.92) = −2.0372, P-value = 0.04384 between ‘I. low’ and ‘II. increasing’; t(86.344) = 0.73256, P-value = 0.4658, where the numbers of sites are 65, 58 and 40, respectively for ‘I. low’, ‘II. increasing’, and ‘III. high’. * indicates p-value ≤ 0.05.
Supplementary Figure 4 mRNA expression levels of ADAR enzymes in different tissues.
In each tissue, fetal and adult samples are compared: (a) ADAR1 (b) ADAR2. Note that boxplots are generated for visualization, with 3 fetal and 3 adult samples in every tissue, except for adult heart (4 samples) and fetal liver (2 samples) tissues.
Supplementary Figure 5 Genome-wide editing rate differences between fetal and adult samples in multiple tissues.
We modified our tool to identify RNA editing sites for a simple but thorough approach to compare RNA editing rates between fetal and adult samples as follows: First, we made an initial call of RNA editing sites, which include the sites with at least five sequencing reads with at least two variant-supporting reads. Second, the possible genomic variants were removed by excluding SNP sites (except for SNPs of molecular type ‘cDNA’), sites only shown in a single sample, and sites with multiple variants (by removing those sites whose numbers of sequencing reads supporting the major and the minor allele are less than 95% of total sequencing reads). Finally, we compared A-to-I editing rates between fetal and adult samples if the median depth of a site is greater than 20 and a site is in mRNA regions (5´UTR, CDS and 3´UTR). (a) Histogram of mean editing rate differences between fetal and adult samples. Mean editing rate difference is defined by the mean editing rate of adult samples minus the mean editing rate of fetal samples. The blue is used to describe a site whose mean editing rate is greater in fetal compared to adult while the red indicates the opposite. (b) Venn diagram showing the overlap of sites whose editing rate differences are greater than 0.2 in lung, liver and brain tissues. Numbers indicate the number of editing sites.
Supplementary Figure 6 Increasing pattern in mouse brain development.
(a) Among 742 A-to-I editing sites showing the increasing pattern in human brain, 95 sites are conserved in the mouse genome (UCSC mm10). Among them, 64 sites with adequate sequencing depths to reliably estimate editing rates (median sequencing depths greater than 20) are described. The conserved A-to-I editing sites are classified according to clusters of increasing pattern (Supplementary Fig. 1b) and gene regions (CDS, 3´UTR). The conserved A-to-I editing sites showed increasing editing patterns, mainly in CDS regions. Dot size is proportional to three categories of sequencing depth: low, ‘L’ (less than 20), medium, ‘M’ (20 to 50), high, ‘H’ (greater than 50). (b) mRNA expression levels of Adar1 (blue) and Adar2 (purple) in mouse brain development. Lines are generated by locally weighted scatterplot smoothing (LOESS) regression with shades indicating 95% confidence interval. (c) Schematic diagram of gene structure for Neil1: a box and a line represent an exon and an intron, respectively. Neil1 is a single exceptional site in a CDS, showing marked loss of the increasing pattern, which may be understood in terms of Alu repeats in the human genome. The Alu repeats neighboring this site form long double-stranded structures and are believed to induce A-to-I editing only in human tissues (Daniel, C. et al., reference 34 in the main text). A double-stranded structure is depicted by arcs, which connect two base-paired nucleotides (‘baseparing arcs’) using an R library, R-CHIE (www.e-rna.org/r-chie). A-to-I editing sites with two different patterns are found in NEIL1: a green (increasing) and a red (stable high).
Supplementary Figure 7 Increasing pattern normalized by neuronal or glial proportions in human brain development.
(a) The proportions of four cell types including embryonic stem cells (ES), neural progenitor cells (NPC), neuronal cells (NeuN pos) and glial cells (NeuN Neg) were estimated from the same brain tissues in the 33 discovery set except for fetal samples (see methods). We used two independent fetal tissues that have both DNA methylation and RNA-seq datasets. Both are samples from 19 weeks. (b) Editing rates normalized by glial proportion (c) Editing rates normalized by the neuronal proportion (d) Editing rates normalized by the ratio of neuronal proportion to glial proportion. In b, c and d, lines indicate the five increasing clusters defined in figure 1c and supplementary figure 1b (The same colors and the same numbers are used across the figures). Lines are generated by LOESS regression of editing rates with 95% confidence interval indicated by shades.
Supplementary Figure 8 Editing rates in fetal and adult neurons.
Editing rates at the representative sites for three editing patterns are compared between the pooled (N=110) fetal and the pooled (N=130) adult neurons. Only sites, denoted by dots, whose sequencing depths are greater than 20 are used. Two-sided t-tests (Welch) were performed for each pattern group: (I. low) N=55, 57, t(99.402) = −2.2981, P-value = 0.02365 (II. increasing) N=41, 47, t(61.931) = −11.151, P-value<2.2e-16 (III. high) N=13, 22, t(20.307) = −1.3063, P-value = 0.2061, where N is the number of editing sites in fetal and adult samples, respectively. The same colors from Figure 2a are used to indicate the three developmental pattern groups: ‘Group I. low’ (blue), ‘Group II. increasing’ (green), ‘Group III. high’ (magenta). **** and * indicate P-value ≤0.0001 and P-value ≤0.05 in two-sided t-tests, respectively.
Supplementary Figure 9 Comparison of ADAR mRNA level across cell types.
Normalized counts of sequencing reads from a previous single cell RNA-seq dataset (Darmanis, S. et al., reference 23 in the main text) were used to compare mRNA levels between neurons and different cell types. Each dot represents a single cell. (A) ADAR1 (B) ADAR2. Two-sided t-tests (Welch) were performed in the following comparisons. For ADAR1, fetal neuron vs. astrocyte: t(129.41) = −1.2582, P-value = 0.2106; fetal neuron vs. oligodendrocyte: t(70.329) = 1.61, P-value = 0.1119; fetal neuron vs. microglia: t(20.94) = 3.1076, P-value = 0.005341; adult neuron vs. astrocyte: t(105.05) = −3.3094, P-value = 0.0009247; adult neuron vs. oligodendrocyte: t(56.54) = 3.503, P-value = 0.0009067; adult neuron vs. microglia: t(18.408) = −4.4675, P-value = 0.0002831. In ADAR2, fetal neuron vs. astrocyte: t(157.33) = −1.5392, P-value = 0.1258; fetal neuron vs. oligodendrocyte: t(109.59) = 4.2221, P-value = 5.015e-5; fetal neuron vs. microglia: t(122.46) =3.3545, P-value = 0.001059; adult neuron vs. astrocyte: t(170.4) = −3.4253, P-value = 0.0007696; adult neuron vs. oligodendrocyte: t(129.66) = 6.7375, P-value = 4.757e-10; adult neuron vs. microglia: t(140.9) = −5.7092, P-value = 6.464e-8. Number of cells are 110 (fetal neurons), 130 (adult neurons), 62 (astrocytes), 38 (oligodendrocytes), 16 (microglia). ****, *** and ** indicate P-value ≤0.0001, P-value ≤0.001 and P-value ≤0.01 in two-sided t-tests, respectively.
Supplementary Figure 10 Editing rate differences among brain regions.
A-to-I editing sites showing significant differences between cerebellum (CB) and other brain regions (DLPFC: dorsolateral prefrontal cortex, HIPPO: hippocampus, ERC: entorhinal cortex) are described. Each line corresponds to an editing site and the associated gene name is shown. (a) Editing sites whose editing rates are higher in CB. (b) Editing sites with higher editing rates in neocortical regions. Lines are generated by LOESS regression of editing rates with 95% confidence interval indicated by shades.
Supplementary Figure 11 Increasing patterns in in vitro differentiation of embryonic stem cells into cortical neurons.
(a) The magnitude of editing rate changes found at the increasingly edited sites. The magnitude of editing rate change is defined as the mean editing rate difference between fetal and post-infant samples for human brain development while it is defined as the mean editing rate difference between samples of in vitro day 0 and 7 and samples of in vitro day 33 and 49 for hESC samples. The x-axis numbers indicate the clusters, which is the same as the numbers shown in the figure 1c, supplementary figure 1b and the figure 4b. A dot corresponds to an editing site. (b) Editing rate change in in vitro differentiation of mESCs to cortical neurons. Different colors indicate different clusters in supplementary figure 1b to show conserved relative editing rates within the increasing pattern. ‘Days In-vitro (DIV)’ corresponds to the following descriptions, which are adapted from Hubbard, K. S. et al. (reference 25 in the main text): DIV -8, embryonic stem cells; DIV -4, neuroepithelial stem cells; DIV 0, radial glial cells; DIV 1, developmental stage (DS) I/II neurons; DIV 7, DS III/IV neurons; DIV 16,21,28, maturing DS IV/V neurons. Dot shape is proportional to three categories of sequencing depth: low (less than 20), medium (20 to 50), high (greater than 50).
Supplementary Figure 12 Cellular markers in in vitro differentiation of hESCs to cortical neurons.
Expression levels of markers measured by RNA-seq with an RPKM unit: POU5F1 (OCT4) and N ANOG for pluripotency, PAX6 and S OX1 for neural differentiation, EMX2 and OTX2 for cortical specification, TBR1 and BCL11B (CTIP2) for deep layer neurons, CACN A1E for upper layer neurons, GFAP for glial cells, MAP2 for dendrites, MAPT for axons. Lines are generated by LOESS regression of editing rates with shades indicating 95% confidence interval.
Supplementary Figure 13 Cellular markers in primary culture of mouse cortical neurons.
Expression levels of markers are measured by RNA-seq with an RPKM unit: Rbfox3 (NeuN) for neurons, Gfap for glial cells, Syn1 and Syn2 for synaptic formation, Map2 for dendrites, Mapt for axons.
Supplementary Figure 14 Correlation of editing rates with mRNA expression levels.
Spearman correlation coefficient between editing rates and mRNA expression levels are calculated at the sites showing the increasing pattern. When there are multiple editing sites in a gene, the highest correlation value is assigned to a gene. (a) Distribution of correlation coefficients at the total sites with the increasing pattern (b) Distributions of correlation coefficients are separately described according to the two gene regions associated with the sites: CDS and 3´ UTR. These distributions are significantly different each other (N=23, 211 for CDS and 3´ UTR, respectively): D=0.3198, P-value = 0.02875 by Kolmogorov-Smirnov test.
Supplementary Figure 15 Editing rates in a glioblastoma and a matched control.
Editing sites in the three groups of selected sites in Figure 2a are compared between glioblastoma tissue and neighboring a non-tumor tissue. Color denotes editing group same as in Figure 2a: ‘Group I: low’ (blue), ‘Group II: increasing’ (green) and ‘Group III: high’ (magenta). Color shade is proportional to sequencing depth: low (less than 20), medium (20 to 50), high (greater than 50). These samples were obtained from an independent patient from Figure 4f.
Supplementary information
Supplementary Text and Figures
Supplementary figures 1–15 (PDF 2009 kb)
Supplementary Software
R and python source codes that used to analyze RNA editing in 33 human brain samples (ZIP 5 kb)
Rights and permissions
About this article
Cite this article
Hwang, T., Park, CK., Leung, A. et al. Dynamic regulation of RNA editing in human brain development and disease. Nat Neurosci 19, 1093–1099 (2016). https://doi.org/10.1038/nn.4337
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4337
This article is cited by
-
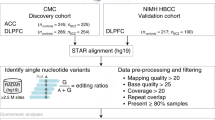
Transcriptomic analysis reveals associations of blood-based A-to-I editing with Parkinson’s disease
Journal of Neurology (2024)
-
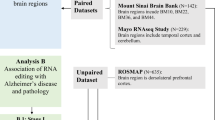
The Q/R editing site of AMPA receptor GluA2 subunit acts as an epigenetic switch regulating dendritic spines, neurodegeneration and cognitive deficits in Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Changes in ADAR RNA editing patterns in CMV and ZIKV congenital infections
BMC Genomics (2023)
-
Deep transcriptome profiling reveals limited conservation of A-to-I RNA editing in Xenopus
BMC Biology (2023)
-
The role of post-transcriptional modifications during development
Biologia Futura (2023)