Abstract
We compared the dynamics of hippocampal and prefrontal interactions in rats as they used spatial contexts to guide the retrieval of object memories. Functional connectivity analysis indicated a flow of contextual information from the hippocampus to prefrontal cortex upon the rat's entry into the spatial context. Conversely, upon the onset of object sampling, the direction of information flow reversed, consistent with prefrontal control over the retrieval of context-appropriate hippocampal memory representations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Eichenbaum, H. Nat. Rev. Neurosci. 15, 732–744 (2014).
Kuhl, B.A. & Wagner, A.D. in Encyclopedia of Neuroscience, Vol. 9 (eds. L.R. Squire et al.) 437–444 (Academic Press, 2009).
Szczepanski, S.M. & Knight, R.T. Neuron 83, 1002–1018 (2014).
Farovik, A., Dupont, L.M., Arce, M. & Eichenbaum, H. J. Neurosci. 28, 13428–13434 (2008).
Navawongse, R. & Eichenbaum, H. J. Neurosci. 33, 1002–1013 (2013).
Siapas, A.G., Lubenov, E.V. & Wilson, M.A. Neuron 46, 141–151 (2005).
Hyman, J.M., Zilli, E.A., Paley, A.M. & Hasselmo, M.E. Hippocampus 15, 739–749 (2005).
Benchenane, K. et al. Neuron 66, 921–936 (2010).
Spellman, T. et al. Nature 522, 309–314 (2015).
Anderson, K.L., Rajagovindan, R., Ghacibeh, G.A., Meador, K.J. & Ding, M. Cereb. Cortex 20, 1604–1612 (2010).
Nyhus, E. & Curran, T. Neurosci. Biobehav. Rev. 34, 1023–1035 (2010).
Buzsáki, G., Anastassiou, C.A. & Koch, C. Nat. Rev. Neurosci. 13, 407–420 (2012).
Adhikari, A., Sigurdsson, T., Topiwala, M.A. & Gordon, J.A. J. Neurosci. Methods 191, 191–200 (2010).
Csicsvari, J., Jamieson, B., Wise, K.D. & Buzsáki, G. Neuron 37, 311–322 (2003).
Buzsáki, G. & Wang, X.J. Annu. Rev. Neurosci. 35, 203–225 (2012).
Colgin, L.L. Curr. Opin. Neurobiol. 21, 467–474 (2011).
Watrous, A.J., Fell, J., Ekstrom, A.D. & Axmacher, N. Curr. Opin. Neurobiol. 31, 33–39 (2015).
Canolty, R.T. & Knight, R.T. Trends Cogn. Sci. 14, 506–515 (2010).
Tort, A.B.L., Komorowski, R.W., Manns, J.R., Kopell, N.J. & Eichenbaum, H. Proc. Natl. Acad. Sci. USA 106, 20942–20947 (2009).
Staudigl, T. & Hanslmayr, S. Curr. Biol. 23, 1101–1106 (2013).
Komorowski, R.W., Manns, J.R. & Eichenbaum, H. J. Neurosci. 29, 9918–9929 (2009).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Elsevier, 2006).
Mitra, P.P. & Bokil, H. Observed Brain Dynamics (Oxford Univ. Press, 2008).
Barnett, L. & Seth, A.K. J. Neurosci. Methods 223, 50–68 (2014).
Barrens, P. J. Stat. Softw. 31, http://dx.doi.org/10.18637/jss.v031.i10 (2009).
Acknowledgements
The work was supported by NIMH grants MH 094263 and MH 051570 to H.E.
Author information
Authors and Affiliations
Contributions
All authors designed the experiment. R.P., A.F. and M.B. conducted the experiment. R.P. conduced the data analyses. R.P. and H.E. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
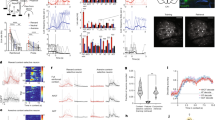
Supplementary Figure 1 Electrode locations and characteristics of local field potentials.
(a) Locations of electrode tips in prefrontal cortex (mPFC), dorsal hippocampus (dHPC), and ventral hippocampus (vHPC) at mm AP to bregma: mPFC 3.20; dHPC 3.14; vHPC -5.60. mPFC electrodes were localized in layers II/III (n=7); dHPC in the pyramidal layer (n=6); vHPC in the pyramidal layer (n=2) or stratum radiatum (n=2). (b) Frequency spectra show prominent power spectral density in the theta band during both the context exploration (Peak Frequency = 8.13 +/− 0.61 Hz, mPFC; 8.93 +/− 0.15 Hz, dHPC; 9.11 +/− 0.15 Hz, vHPC) and object sampling periods (Peak Frequency = mPFC: 8.3 +/− 0.43 Hz; dHPC: 8.79 +/− 0.14 Hz; vHPC: 9.03 +/− 0.13 Hz). (c) Mean coherence between dHPC and vHPC and mPFC was maximal at theta frequency both for the context exploration (Peak Frequency = dHPC-mPFC: 9.57 Hz +/−0.17 Hz; vHPC-mPFC: 9.51 +/− 0.23 Hz) and object sampling periods (Peak Frequency = dHPC-mPFC: 9.34 +/− 0.21; vHPC-mPFC: 8.91 +/− 0.14 Hz). Shading indicates S.E.M. (d) Peak power frequency during first 1-s of context exploration and object sampling did not significantly differ among areas (One-way ANOVA F1, 30 = 0, p = 0.97 +/− S.E.M. (e). Peak Coherence Frequency during first 1 s of context exploration and versus object sampling did not significantly differ (One-way ANOVA, F1, 18 = 3.49, p = 0.08). Coherence values for these frequencies were greater than those predicted by 95% jackknife-estimated confidence intervals (0.1069) for both context exploration (Coherency = dHPC-mPFC: 0.47 +/− 0.04; vHPC-mPFC: 0.38 +/− 0.02) and object sampling (Coherency = dHPC-mPFC: 0.42 +/− 0.05; vHPC-mPFC: 0.32 +/− 0.02).
Supplementary Figure 2 Normalized correlations between instantaneous theta amplitude across a range of shifts between LFPs recorded in hippocampus (HPC) and prefrontal cortex (mPFC) for individual subjects.
Correlation patterns for post-learning sessions (a,b) and for correct trials (c,d) and errors (e,f) during learning sessions.
Supplementary Figure 3 Granger causal (GC) relationships (1–25 Hz) across sessions for context exploration and object sampling.
GC was greater from HPC to mPFC during (a) context exploration (Two-way ANOVA, F = 1033.46, df = 1, p = 9.83e-215), while mPFC better predicted HPC activity during instances of (b) object sampling (Two-way ANOVA, F = 2571.27, df = 1, p < 0.0001). Furthermore, maximal GC was within the theta range for both context exploration (9.16 +/− 0.83 Hz) and object sampling periods (9.27 +/− 0.92 Hz), and the causal flow reversed within the HPC-PFC circuit at this frequency as a function of behavioral phase, such that HPC directed mPFC activity during context exploration (GC = 0.3983 +/− 0.07, Wilcoxon rank-sum, z42 = 4.60, p = 4.15e-6) and mPFC engaged HPC during object sampling (GC = 0.3518 +/− 0.04, Wilcoxon rank-sum, z42 = 4.96, p = 7.14e-8). Bayesian Information Criterion (BIC) indicated the best fitting model order was at 40 (+/− 2.60) ms and 26 (+/− 2.50) ms lags for context versus object sampling periods respectively. The HPC lead over mPFC observed during contextual exploration is consistent with previously demonstrated Granger causal predictions of HPC-mPFC functional connectivity13. Shaded plots represent S.E.M. across sessions. Inset bar graph: Comparison between GC directionality for frequency of peak GC (mean +/− standard error).
Supplementary Figure 4 Movement speed patterns during context exploration and object sampling.
(a) Speed patterns over the entire 10 sec context exploration period and period surrounding object sampling for each trial in an example session. On individual trials rats rapidly enter the context then alternate irregularly between rapid movements and periods of immobility. During object sampling, rats typically sniff standing still then adjust the head to a new position for additional sampling, such that the changes in speed observed during the object sampling period reflect head turning while standing relatively still examining the object. (b) Movement speed pattern for 1 sec context exploration and object sampling periods averaged (+/− SE) for all sessions. Note that “speed” in these periods reflects qualitatively different behaviors when the animal is walking through space or turning its head while still. (c) Scatter plot of PFC-HPC lag/leads and speed over 1 sec context exploration (context) and object sampling (object) periods from all sessions. Lag/lead did not significantly correlate with speed during the context exploration period (vHPC-mPFC: r = 0.06, p = 0.85; dHPC-mPFC: r = 0.009, p = 0.97; combined HPC-mPFC: Pearson r = -0.008, p = 0.96) or the object exploration period (vHPC-mPFC: r = -0.33, p = 0.28; dHPC-mPFC: r = 0.413, p = 0.08; combined HPC-mPFC: r = 0.023, p = 0.90). Note the r-value closest to significance (p = 0.08) hints at a positive correlation suggesting greater mPFC lead over dHPC with increasing speed, which is opposite the direction observed during higher speeds associated with context exploration.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Tables 1–3 (PDF 882 kb)
Rights and permissions
About this article
Cite this article
Place, R., Farovik, A., Brockmann, M. et al. Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat Neurosci 19, 992–994 (2016). https://doi.org/10.1038/nn.4327
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4327
This article is cited by
-
Fish oil omega-3 Fatty Acids Alleviate Postoperative delirium-like Behavior in aged mice by Attenuating Neuroinflammation and Oxidative Stress
Neurochemical Research (2024)
-
Theta Oscillations Support Prefrontal-hippocampal Interactions in Sequential Working Memory
Neuroscience Bulletin (2024)
-
Conflict Dynamics of Post-Retrieval Extinction: A Comparative Analysis of Unconditional and Conditional Reminders Using Skin Conductance Responses and EEG
Brain Topography (2024)
-
Thalamic nucleus reuniens coordinates prefrontal-hippocampal synchrony to suppress extinguished fear
Nature Communications (2023)
-
A model of working memory for encoding multiple items and ordered sequences exploiting the theta-gamma code
Cognitive Neurodynamics (2023)