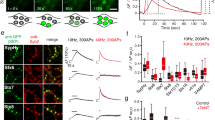
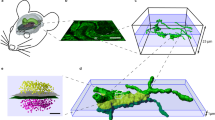
Abstract
Neurotransmission at dopaminergic synapses has been studied with techniques that provide high temporal resolution, but cannot resolve individual synapses. To elucidate the spatial dynamics and heterogeneity of individual dopamine boutons, we developed fluorescent false neurotransmitter 200 (FFN200), a vesicular monoamine transporter 2 (VMAT2) substrate that selectively traces monoamine exocytosis in both neuronal cell culture and brain tissue. By monitoring electrically evoked Ca2+ transients with GCaMP3 and FFN200 release simultaneously, we found that only a small fraction of dopamine boutons that exhibited Ca2+ influx engaged in exocytosis, a result confirmed with activity-dependent loading of the endocytic probe FM1-43. Thus, only a low fraction of striatal dopamine axonal sites with uptake-competent VMAT2 vesicles are capable of transmitter release. This is consistent with the presence of functionally 'silent' dopamine vesicle clusters and represents, to the best of our knowledge, the first report suggestive of presynaptically silent neuromodulatory synapses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zhang, M.Y. & Beyer, C.E. Measurement of neurotransmitters from extracellular fluid in brain by in vivo microdialysis and chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 40, 492–499 (2006).
Schmitz, Y., Lee, C.J., Schmauss, C., Gonon, F. & Sulzer, D. Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J. Neurosci. 21, 5916–5924 (2001).
Kennedy, R.T., Jones, S.R. & Wightman, R.M. Simultaneous measurement of oxygen and dopamine: coupling of oxygen consumption and neurotransmission. Neuroscience 47, 603–612 (1992).
Bérubé-Carrière, N. et al. Ultrastructural characterization of the mesostriatal dopamine innervation in mice, including two mouse lines of conditional VGLUT2 knockout in dopamine neurons. Eur. J. Neurosci. 35, 527–538 (2012).
Pickel, V.M., Beckley, S.C., Joh, T.H. & Reis, D.J. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 225, 373–385 (1981).
Betz, W.J. & Bewick, G.S. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science 255, 200–203 (1992).
Miesenböck, G., De Angelis, D.A. & Rothman, J.E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195 (1998).
Onoa, B., Li, H., Gagnon-Bartsch, J.A., Elias, L.A. & Edwards, R.H. Vesicular monoamine and glutamate transporters select distinct synaptic vesicle recycling pathways. J. Neurosci. 30, 7917–7927 (2010).
Mani, M. & Ryan, T.A. Live imaging of synaptic vesicle release and retrieval in dopaminergic neurons. Front. Neural Circuits 3, 3 (2009).
Pan, P.Y. & Ryan, T.A. Calbindin controls release probability in ventral tegmental area dopamine neurons. Nat. Neurosci. 15, 813–815 (2012).
Daniel, J.A., Galbraith, S., Iacovitti, L., Abdipranoto, A. & Vissel, B. Functional heterogeneity at dopamine release sites. J. Neurosci. 29, 14670–14680 (2009).
Gubernator, N.G. et al. Fluorescent false neurotransmitters visualize dopamine release from individual presynaptic terminals. Science 324, 1441–1444 (2009).
Rodriguez, P.C. et al. Fluorescent dopamine tracer resolves individual dopaminergic synapses and their activity in the brain. Proc. Natl. Acad. Sci. USA 110, 870–875 (2013).
Liu, Y. et al. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell 70, 539–551 (1992).
Matsushita, N. et al. Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J. Neurochem. 82, 295–304 (2002).
Fuxe, K., Hökfelt, T., Olson, L. & Ungerstedt, U. Central monoaminergic pathways with emphasis on their relation to the so called 'extrapyramidal motor system'. Pharmacol. Ther. [B] 3, 169–210 (1977).
Pickel, V.M., Nirenberg, M.J. & Milner, T.A. Ultrastructural view of central catecholaminergic transmission: immunocytochemical localization of synthesizing enzymes, transporters and receptors. J. Neurocytol. 25, 843–856 (1996).
Thompson, L., Barraud, P., Andersson, E., Kirik, D. & Björklund, A. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. J. Neurosci. 25, 6467–6477 (2005).
Caudle, W.M. et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J. Neurosci. 27, 8138–8148 (2007).
Mooslehner, K.A. et al. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism. Mol. Cell. Biol. 21, 5321–5331 (2001).
Grace, A.A. & Bunney, B.S. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 4, 2877–2890 (1984).
Kennedy, R.T., Jones, S.R. & Wightman, R.M. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J. Neurochem. 59, 449–455 (1992).
Abeliovich, A. et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252 (2000).
Arbuthnott, G.W. & Wickens, J. Space, time and dopamine. Trends Neurosci. 30, 62–69 (2007).
Groves, P.M., Linder, J.C. & Young, S.J. 5-hydroxydopamine-labeled dopaminergic axons: three-dimensional reconstructions of axons, synapses and postsynaptic targets in rat neostriatum. Neuroscience 58, 593–604 (1994).
Altrock, W.D. et al. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron 37, 787–800 (2003).
Rosenmund, C. et al. Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron 33, 411–424 (2002).
Moulder, K.L. et al. Plastic elimination of functional glutamate release sites by depolarization. Neuron 42, 423–435 (2004).
Kannenberg, K., Sieghart, W. & Reuter, H. Clusters of GABAA receptors on cultured hippocampal cells correlate only partially with functional synapses. Eur. J. Neurosci. 11, 1256–1264 (1999).
Cousin, M.A. & Evans, G.J. Activation of silent and weak synapses by cAMP-dependent protein kinase in cultured cerebellar granule neurons. J. Physiol. (Lond.) 589, 1943–1955 (2011).
Sgobio, C. et al. Optogenetic measurement of presynaptic calcium transients using conditional genetically encoded calcium indicator expression in dopaminergic neurons. PLoS One 9, e111749 (2014).
Zariwala, H.A. et al. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci. 32, 3131–3141 (2012).
Bäckman, C.M. et al. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis 44, 383–390 (2006).
Zakharenko, S.S., Zablow, L. & Siegelbaum, S.A. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat. Neurosci. 4, 711–717 (2001).
Zhang, H. & Sulzer, D. Frequency-dependent modulation of dopamine release by nicotine. Nat. Neurosci. 7, 581–582 (2004).
Rice, M.E. & Cragg, S.J. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 7, 583–584 (2004).
Jahromi, S.S. & Atwood, H.L. Three-dimensional ultrastructure of the crayfish neuromuscular apparatus. J. Cell Biol. 63, 599–613 (1974).
Hirst, G.D., Redman, S.J. & Wong, K. Post-tetanic potentiation and facilitation of synaptic potentials evoked in cat spinal motoneurones. J. Physiol. (Lond.) 321, 97–109 (1981).
Bennett, M., Jones, P. & Lavidis, N. Transmitter secretion varies between visualized release sites at amphibian neuromuscular junctions. Neurosci. Lett. 65, 311–315 (1986).
Losonczy, A., Biró, A.A. & Nusser, Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc. Natl. Acad. Sci. USA 101, 1362–1367 (2004).
Chavis, P., Mollard, P., Bockaert, J. & Manzoni, O. Visualization of cyclic AMP-regulated presynaptic activity at cerebellar granule cells. Neuron 20, 773–781 (1998).
Moulder, K.L., Jiang, X., Taylor, A.A., Olney, J.W. & Mennerick, S. Physiological activity depresses synaptic function through an effect on vesicle priming. J. Neurosci. 26, 6618–6626 (2006).
Jiang, X. et al. A role for the ubiquitin-proteasome system in activity-dependent presynaptic silencing. J. Neurosci. 30, 1798–1809 (2010).
Matsuda, W. et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 29, 444–453 (2009).
Cacciapaglia, F., Wightman, R.M. & Carelli, R.M. Rapid dopamine signaling differentially modulates distinct microcircuits within the nucleus accumbens during sucrose-directed behavior. J. Neurosci. 31, 13860–13869 (2011).
Robinson, D.L., Howard, E.C., McConnell, S., Gonzales, R.A. & Wightman, R.M. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol. Clin. Exp. Res. 33, 1187–1196 (2009).
Choi, S., Klingauf, J. & Tsien, R.W. Postfusional regulation of cleft glutamate concentration during LTP at 'silent synapses'. Nat. Neurosci. 3, 330–336 (2000).
Ma, L., Zablow, L., Kandel, E.R. & Siegelbaum, S.A. Cyclic AMP induces functional presynaptic boutons in hippocampal CA3-CA1 neuronal cultures. Nat. Neurosci. 2, 24–30 (1999).
Voronin, L.L. et al. Postsynaptic depolarisation enhances transmitter release and causes the appearance of responses at “silent” synapses in rat hippocampus. Neuroscience 126, 45–59 (2004).
Porrill, J. & Dean, P. Silent synapses, LTP, and the indirect parallel-fibre pathway: computational consequences of optimal cerebellar noise-processing. PLoS Comput. Biol. 4, e1000085 (2008).
Adam, Y., Edwards, R.H. & Schuldiner, S. Expression and function of the rat vesicular monoamine transporter 2. Am. J. Physiol. Cell Physiol. 294, C1004–C1011 (2008).
Giros, B., Jaber, M., Jones, S.R., Wightman, R.M. & Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612 (1996).
Mena, M.A. et al. Effects of wild-type and mutated copper/zinc superoxide dismutase on neuronal survival and L-DOPA-induced toxicity in postnatal midbrain culture. J. Neurochem. 69, 21–33 (1997).
Wong, M.Y., Sulzer, D. & Bamford, N.S. Imaging presynaptic exocytosis in corticostriatal slices. Methods Mol. Biol. 793, 363–376 (2011).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 (2009).
Zipfel, W.R., Williams, R.M. & Webb, W.W. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol. 21, 1369–1377 (2003).
Schmitz, Y., Schmauss, C. & Sulzer, D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J. Neurosci. 22, 8002–8009 (2002).
Acknowledgements
We thank M. Caron (Duke University) for providing DAT knockout mice, A. Salahpour (University of Toronto) and G. Miller (Emory University) for the VMAT2 hypomorph, mice and K. Kobayashi (Fukushima Medical University) for the TH-GFP mice. We would also like to thank C. Castagna, V. Morales, A. Barnett and D. Korostyshevsky for excellent technical support as well as other members of the Sulzer and Sames laboratories for helpful discussions and support. This work was supported by the G. Harold & Leila Y. Mathers Charitable Foundation (D. Sames), National Institute on Mental Health (R01MH086545 to D. Sames, R01MH108186 to D. Sames and D. Sulzer), the J.P.B. (D. Sulzer), McKnight (D. Sames and D. Sulzer) and Parkinson's Disease Foundations (D. Sulzer), the National Institute on Drug Abuse (DA07418 and DA10154; D. Sulzer), the National Institute on Alcohol Abuse and Alcoholism (AA019801; D. Sulzer), and a National Institute of Neurological Disorders and Stroke (NINDS) Udall Center of Excellence for Parkinson's Disease Research (D. Sulzer). Y.S. was supported by the Michael J. Fox Foundation, J.E.L.-O. was supported by NINDS (3 P50 NS 038370-13S1), P.C.R. was granted a pre-doctoral fellowship by the National Science Foundation and E.V.M. was funded by NINDS (R01NS075222).
Author information
Authors and Affiliations
Contributions
D.B.P., Y.S., E.V.M., D. Sulzer and D. Sames conceived and designed the experiments. S.L. conducted the early examination of FFN200 in brain tissue. D.B.P., P.M. and P.C.R. performed slice imaging probe characterization experiments and Y.S. and E.V.M. performed cell culture imaging experiments. D.B.P. performed brain slice FFN200 destaining experiments, including FFN200-GCaMP3 simultaneous imaging. J.M., aided by D.B.P., developed the Matlab image analysis routine and T.J.M. contributed with additional data output scripts. D. Sames designed FFN200, and G.H. and A.H. designed and performed FFN200 synthesis and purification as well as chemical and photophysical characterization. R.J.K. performed the FFN200 Km determination experiments. J.E.L.-O. designed and performed the CV experiments. M.S.S. aided with the FFN200-GCaMP3 simultaneous imaging experiments. E.K. optimized and provided midbrain dopamine cultures. D.B.P., Y.S., P.M., J.E.L.-O., R.J.K., P.C.R. and E.V.M. analyzed data. D.B.P. wrote the paper, with important contributions from Y.S., J.M., M.S.S., E.V.M., P.C.R., D. Sulzer and D. Sames.
Corresponding authors
Ethics declarations
Competing interests
D. Sulzer and D. Sames were listed as inventors on a patent (8,337,941) covering FFN200 and a patent application (13/575,535) covering FFN102, compounds employed in this study.
Integrated supplementary information
Supplementary Figure 1 FFN200 accumulates in cultured dopamine neurons.
(a) Representative images of FFN200-labeled dopamine ventral midbrain neurons obtained from TH-GFP mice, displaying different levels of FFN200 accumulation following incubation with 10 μM FFN200 for 30 min at 37°C. (b) Scatter plot of FFN200 intensity values at GFP-positive dopamine neurons, presented in ascending order in a logarithmic scale. Neurons were considered FFN200-positive when their fluorescence intensity was greater than two standard deviations above the mean FFN200 intensity of GFP-negative cells (the threshold is depicted as a dotted line; 80 cells from six dishes, four independent cultures). An example of an FFN200-negative neuron is shown in the top TH-GFP/FFN200 image pair in panel (a), and the following two image pairs show FFN200-positive neurons.
Supplementary Figure 2 Effect of dTBZ on dopamine release as a function of incubation time.
Single pulse-evoked dopamine release was measured by cyclic voltammetry in the dorsal striatum of control and 5 μM dTBZ-treated slices (n=3–4 mice with 1–2 slices averaged per mouse). Released dopamine was normalized for each condition to the average current of five prepulses applied before time point zero, which marks the beginning of dTBZ perfusion (in dTBZ-treated slices).
Supplementary Figure 3 Effect of 0 mM Ca2+ and TTX on FFN200 release in the dorsal striatum.
(a) Background intensity presented as mean percentage of Fi ± SEM for slices stimulated in the presence of 2.4 mM Ca2+, in the absence of Ca2+ (0 mM Ca2+) or in 2.4 mM Ca2+ with 1 μM TTX (2.4 mM Ca2+ + TTX; n=6 for each condition in both panels, where n is number of mice, with 1–2 slices averaged per mouse). (b) Scatter plot of the percentage of destaining puncta in each independent experiment including mean ± SEM (**p < 0.01 by one-way ANOVA with Bonferroni's multiple comparison test).
Supplementary Figure 4 Comparison of FFN200 and FFN102 release in the dorsal striatum.
(a) Background intensity presented as mean percentage of Fi ± SEM for both probes (n=9 and 8 slices from different mice for FFN200 and FFN102, respectively, for all panels in this figure). (b) Scatter plot of the percentage of FFN200 and FFN102 destaining puncta in response to 15 Hz stimuli in each independent experiment including mean ± SEM (n.s- not significantly different, p=0.1269, two-tailed unpaired t test). As a control, the percentage of FFN102 puncta selected as “destainers” in unstimulated slices was 5.1 ± 1.6% (n=5; not depicted). (c) FFN200 and FFN102 puncta intensity (background- and baseline rundown-corrected) over time, normalized to ΔF, the fluorescence change between the last point of the baseline (100%) and the average of the last three data points of stimulation (0%) ± SEM, to facilitate comparison between FFN200 and FFN102 curves (*p < 0.05, two-tailed unpaired t test; p=0.0370). (d) Cumulative distribution of t1/2s of destaining for FFN200 and FFN102 (147 and 175 puncta from nine and eight slices from different mice for FFN200 and FFN102, respectively).
Supplementary Figure 5 Effects of short washout times on FFN200 release in the dorsal striatum.
(a) Scatter plot of the percentage of destaining puncta in each independent experiment, including mean ± SEM, for typical 45 min washout (45’ washout) and shorter 25 min washout (25’ washout) (n=6 for both conditions in both panels, where n is number of mice, with 1–2 slices averaged per mouse; n.s- not significantly different, p=0.4771 by two-tailed unpaired t test). (b) Puncta fluorescence intensity (background- and baseline rundown-corrected) over time presented as mean percentage of Fi ± SEM.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 576 kb)
Rights and permissions
About this article
Cite this article
Pereira, D., Schmitz, Y., Mészáros, J. et al. Fluorescent false neurotransmitter reveals functionally silent dopamine vesicle clusters in the striatum. Nat Neurosci 19, 578–586 (2016). https://doi.org/10.1038/nn.4252
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4252
This article is cited by
-
Striatal dopamine neurotransmission is altered in age- and region-specific manner in a Parkinson’s disease transgenic mouse
Scientific Reports (2024)
-
Development, wiring and function of dopamine neuron subtypes
Nature Reviews Neuroscience (2023)
-
Acetylcholine waves and dopamine release in the striatum
Nature Communications (2023)
-
Inhibition of LRRK2 kinase activity rescues deficits in striatal dopamine physiology in VPS35 p.D620N knock-in mice
npj Parkinson's Disease (2023)
-
A synaptomic analysis reveals dopamine hub synapses in the mouse striatum
Nature Communications (2022)