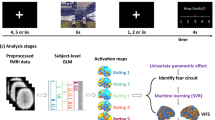
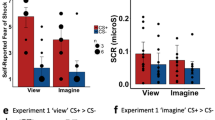
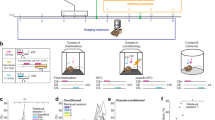
Abstract
Organisms tend to respond similarly to stimuli that are perceptually close to an event that predicts adversity, a phenomenon known as fear generalization. Greater dissimilarity yields weaker behavioral responses, forming a fear-tuning profile. The perceptual model of fear generalization assumes that behavioral fear tuning results from perceptual similarities, suggesting that brain responses should also exhibit the same fear-tuning profile. Using fMRI and a circular fear-generalization procedure, we tested this prediction. In contrast with the perceptual model, insula responses showed less generalization than behavioral responses and encoded the aversive quality of the conditioned stimulus, as shown by high pattern similarity between the conditioned stimulus and the shock. Also inconsistent with the perceptual model, object-sensitive visual areas responded to ambiguity-related outcome uncertainty. Together these results indicate that fear generalization is not passively driven by perception, but is an active process integrating threat identification and ambiguity-based uncertainty to orchestrate a flexible, adaptive fear response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pavlov, I. Conditioned Reflexes: an Investigation of the Physiological Activity of the Cerebral Cortex (Oxford University Press Humphrey Milford, 1927).
Bass, M.J. & Hull, C.L. The irradiation of a tactile conditioned reflex in man. J. Comp. Psychol. 17, 47–65 (1934).
Guttman, N. & Kalish, H.I. Discriminability and stimulus generalization. J. Exp. Psychol. 51, 79–88 (1956).
Ghirlanda, S. & Enquist, M. A century of generalization. Anim. Behav. 66, 15–36 (2003).
Shepard, R.N. Toward a universal law of generalization for psychological science. Science 237, 1317–1323 (1987).
Tenenbaum, J.B. & Griffiths, T.L. Generalization, similarity, and Bayesian inference. Behav. Brain Sci. 24, 629–640 (2001).
Lissek, S. et al. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav. Res. Ther. 46, 678–687 (2008).
Resnik, J., Sobel, N. & Paz, R. Auditory aversive learning increases discrimination thresholds. Nat. Neurosci. 14, 791–796 (2011).
Schechtman, E., Laufer, O. & Paz, R. Negative valence widens generalization of learning. J. Neurosci. 30, 10460–10464 (2010).
Holt, D.J. et al. A parametric study of fear generalization to faces and non-face objects: relationship to discrimination thresholds. Front. Hum. Neurosci. 8, 624 (2014).
Dunsmoor, J.E., Prince, S.E., Murty, V.P., Kragel, P.A. & LaBar, K.S. Neurobehavioral mechanisms of human fear generalization. Neuroimage 55, 1878–1888 (2011).
Dunsmoor, J.E. & Murphy, G.L. Categories, concepts, and conditioning: how humans generalize fear. Trends Cogn. Sci. 19, 73–77 (2015).
Dymond, S., Dunsmoor, J.E., Vervliet, B., Roche, B. & Hermans, D. Fear generalization in humans: systematic review and implications for anxiety disorder research. Behav. Ther. 26, 561–582 (2015).
Lissek, S. et al. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am. J. Psychiatry 167, 47–55 (2010).
Cha, J. et al. Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: implications for generalized anxiety disorder. J. Neurosci. 34, 4043–4053 (2014).
Lissek, S. et al. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol. Psychiatry 75, 909–915 (2014).
Greenberg, T., Carlson, J.M., Cha, J., Hajcak, G. & Mujica-Parodi, L.R. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress. Anxiety 30, 242–250 (2013).
Charney, D.S., Deutch, A.Y., Krystal, J.H., Southwick, S.M. & Davis, M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch. Gen. Psychiatry 50, 295–305 (1993).
Lissek, S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depress. Anxiety 29, 257–263 (2012).
Lissek, S. et al. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc. Cogn. Affect. Neurosci. 9, 1134–1142 (2014).
Grupe, D.W. & Nitschke, J.B. Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion 11, 413–424 (2011).
Lake, J.I. & LaBar, K.S. Unpredictability and uncertainty in anxiety: a new direction for emotional timing research. Front. Integr. Neurosci. 5, 55 (2011).
Grupe, D.W. & Nitschke, J.B. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 14, 488–501 (2013).
Grillon, C., Baas, J.P., Lissek, S., Smith, K. & Milstein, J. Anxious responses to predictable and unpredictable aversive events. Behav. Neurosci. 118, 916–924 (2004).
Hsu, M., Bhatt, M., Adolphs, R., Tranel, D. & Camerer, C.F. Neural systems responding to degrees of uncertainty in human decision-making. Science 310, 1680–1683 (2005).
Nader, K. & Balleine, B. Ambiguity and anxiety: when a glass half full is empty. Nat. Neurosci. 10, 807–808 (2007).
Kanwisher, N., McDermott, J. & Chun, M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 17, 4302–4311 (1997).
Haxby, J.V. et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293, 2425–2430 (2001).
Greenberg, T., Carlson, J.M., Cha, J., Hajcak, G. & Mujica-Parodi, L.R. Neural reactivity tracks fear generalization gradients. Biol. Psychol. 92, 2–8 (2013).
Roy, M., Shohamy, D. & Wager, T.D. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn. Sci. 16, 147–156 (2012).
Dunsmoor, J.E., Kragel, P.A., Martin, A. & LaBar, K.S. Aversive learning modulates cortical representations of object categories. Cereb. Cortex 24, 2859–2872 (2014).
Kriegeskorte, N. Representational similarity analysis – connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2 4 10.3389/neuro.06.004.2008 (2008).
Visser, R.M., Scholte, H.S. & Kindt, M. Associative learning increases trial-by-trial similarity of BOLD-MRI patterns. J. Neurosci. 31, 12021–12028 (2011).
Edeline, J.M. & Weinberger, N.M. Receptive field plasticity in the auditory cortex during frequency discrimination training: selective retuning independent of task difficulty. Behav. Neurosci. 107, 82–103 (1993).
Whalen, P.J. et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J. Neurosci. 18, 411–418 (1998).
Craig, A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666 (2002).
Paulus, M.P. & Stein, M.B. An insular view of anxiety. Biol. Psychiatry 60, 383–387 (2006).
Etkin, A. & Wager, T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in ptsd, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488 (2007).
Kriegeskorte, N., Formisano, E., Sorger, B. & Goebel, R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc. Natl. Acad. Sci. USA 104, 20600–20605 (2007).
Herry, C. et al. Processing of temporal unpredictability in human and animal amygdala. J. Neurosci. 27, 5958–5966 (2007).
Badia, P., Harsh, J. & Abbott, B. Choosing between predictable and unpredictable shock conditions: Data and theory. Psychol. Bull. 86, 1107–1131 (1979).
Grillon, C. et al. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol. Psychiatry 66, 47–53 (2009).
Büchel, C. & Dolan, R.J. Classical fear conditioning in functional neuroimaging. Curr. Opin. Neurobiol. 10, 219–223 (2000).
LaBar, K.S., Gatenby, J.C., Gore, J.C., LeDoux, J.E. & Phelps, E.A. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20, 937–945 (1998).
Büchel, C., Morris, J., Dolan, R.J. & Friston, K.J. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 20, 947–957 (1998).
Maloney, L.T. & Yang, J.N. Maximum likelihood difference scaling. J. Vis. 3, 573–585 (2003).
Watson, A.B. & Pelli, D.G. QUEST: a Bayesian adaptive psychometric method. Percept. Psychophys. 33, 113–120 (1983).
Aguirre, G.K., Mattar, M.G. & Magis-Weinberg, L. de Bruijn cycles for neural decoding. Neuroimage 56, 1293–1300 (2011).
Pelli, D.G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442 (1997).
Benedek, M. & Kaernbach, C. Decomposition of skin conductance data by means of nonnegative deconvolution. Psychophysiology 47, 647–658 (2010).
Mumford, J.A., Poline, J.-B. & Poldrack, R.A. Orthogonalization of regressors in fMRI models. PLoS ONE 10, e0126255 (2015).
Kruschke, J.K. Doing Bayesian Data Analysis: a Tutorial with R and BUGS (Academic Press, 2011).
Desmond, J.E. & Glover, G.H. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J. Neurosci. Methods 118, 115–128 (2002).
Efron, B. & Tibshirani, R. An Introduction to the Bootstrap (Chapman & Hall, 1994).
Acknowledgements
We thank S. Schall, J. Caplan, S. Geuter, T. Kietzmann, A. Etkin, B. Knutson and K. Friston for invaluable comments, and L. Kampermann for her assistance during the data acquisition. S.O. and C.B. are supported by the DFG SFB TRR 58.
Author information
Authors and Affiliations
Contributions
S.O. and C.B. conceived and designed the study. S.O. acquired the data. S.O. and C.B. analyzed and interpreted the data. S.O. drafted the manuscript. C.B. and S.O. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Perceptual organization of face stimuli.
(a) Eight faces forming a circular similarity continuum along gender (x-axis) and identity dimensions (y-axis) (Right: most male face; Left: most female; Top/Bottom: two different facial identities). (b) Recovered perceptual organization of the stimulus set in a two-dimensional perceptual space. Distances between nodes (black dots) represent perceptual similarity between faces that are most likely to have caused the observed binary responses during the two-alternative forced choice task. Ellipses represent 95% chi-square confidence intervals (p =.05), computed following an affine inter-subject alignment. (c) The same perceptual analysis conducted after aligning faces with respect to the CS+ face (red: CS+; cyan: CS–). Circular color code represents the angular distance from the CS+ face. Distances (black arrows) between the CS+ (red node) and all other faces were used to estimate the perceptual tuning at the single-subject level shown in (e). (d) The negative log-likelihood of one-, two- or three-dimensional perceptual models averaged across subjects (mean ± SEM). Decreasing values indicate higher probability for the model. A one-dimensional perceptual model performed worse than the two- and three-dimensional models, whereas the increase from two- to three-dimensions was negligible. (e) Colored bars represent the estimated perceptual distances (shown with black lines in (c)) between the CS+ face and all other faces for two volunteers.
Supplementary Figure 2 Organization of single trials.
(a) The temporal structure of a single trial ending with an UCS delivery. (b) Oddball stimuli consisted of a randomly selected (each time anew) face with artificially added freckles (TR: repetition time; UCS: Unconditional stimulus).
Supplementary Figure 3 Computation of single-subject autonomic fear generalization profiles using skin conductance responses.
(a) From raw electrodermal recordings (not shown), we extracted the phasic skin conductance responses (SCR) using a deconvolution technique50 on a single-subject basis. Time-course of evoked skin conductance responses, averaged across trials during the test phase is depicted for a representative subject. Note that stimulus presentation lasted 1.5 seconds. We computed generalization profiles by averaging the signal within the temporal interval of interest (vertical gray dashed lines) separately for each of the eight individual faces. (b) Fear-tuning from two volunteers. Left panel: An example of wide autonomic fear-tuning (σ =.93 radians) derived from the data shown in (a). Right panel: Fear-tuning from another volunteer exhibiting sharper fear-tuning (σ =.57 radians). Error bars: SEM across trials. Black curves: Best fitting Gaussian model (p <.05, likelihood-ratio test).
Supplementary Figure 4 Brain areas with Gaussian fear tuning.
(a) Horizontal sections showing statistically significant clusters that exhibited fear tuning consistent with a Gaussian model (P <.05, corrected). (b) Fear tuning profiles of peak-voxels within each cluster (depicted in a) are shown for baseline (gray bars) and test phases (red bars, Mean ± SEM). Red curves (Gaussian model) and black horizontal lines (null model) represent the best-fitting model for each phase. In all cases the Gaussian model performed better than the null model only during the test phase but not before. These profiles are sorted from left to right with decreasing selectivity, that is with increasing tuning-width parameter. We refer to the two loci located in the insular region as anterior insula (aIC, shown in the left panel in a) and frontal operculum (shown in the second panel from left in a). These were the only brain areas with a positive amplitude parameter (first and fifth panels in b). (L: Left; R: Right; A: Anterior; P: Posterior).
Supplementary Figure 5 Anterior insula (aIC) is more selective than behavioral and autonomic response profiles.
(a) Posterior distribution of the tuning-width hyper-parameter (σaIC) fitted to generalization profiles recorded at aIC during the test phase. Posterior distributions were obtained using a hierarchical Bayesian model-fitting scheme. Red dotted lines indicate the average value of posterior distributions for σSCR and σRating parameters representing the tuning-width of SCR and shock expectancy ratings. Values correspond to 94th and 98.2th percentiles, respectively. These distributions are shown in (b) in cyan and brown colors, respectively. The average of these three posterior distributions was 0.46 for aIC, 0.60 for SCR and 0.67 for expectancy ratings. (c) Pair-wise differences of single-subject tuning-width parameter between aIC and SCR (left bar) and Ratings (right bar, Mean ± SEM). Positive values indicate smaller tuning-width parameters for aIC. Note that even though single subject parameters are assumed to originate from the same hyper-parameter distribution (shown in (a) and (b)), the pair-wise differences between different sources can be assumed to be independent (t-test (28) = 8.02 and 7.58; p <.001; SCR: skin conductance responses; aIC: anterior insular cortex).
Supplementary Figure 6 Correlation between sharpening index (SI) and neuronal/autonomic tuning parameters.
(a-c) Relationship between SI and the tuning strength (α) of anterior insular cortex (aIC, a), infero-temporal cortex (ITC, b) and ventromedial prefrontal cortex (vmPFC, c). Each point represents a single subject (n = 29). For vmPFC the relationship between SI and tuning-specificity is also shown (d). In the case of ITC, α parameter represents the tuning strength of the cosine model, whereas for all other areas it is the amplitude of the Gaussian model. (e,f) Horizontal lines inside vertical bars depict the correlation between SI and different tuning parameters (e: tuning-strength; f: tuning-specificity) for all areas that exhibited significant fear tuning (listed in Supplementary Table 1). The vertical extent of the bars represents bootstrap confidence intervals at alpha = 0.01 (significant correlations are shown in red). Correlation between fear-tuning parameters of SCR, as well as perceptual tuning (Percept. B, Percept. T: Perceptual tuning before baseline and following test phase, respectively) is also shown. Tuning-specificity arising from the integration of fear responses in aIC and ITC was significantly correlated with SI (last column in f).
Supplementary Figure 7 Evoked multivariate activity patterns within insular region of interest (ROI).
(a) Top panel: Insular ROI for the representational similarity analysis. Bottom panel: Same ROI as a binary glass brain. (b) Average-intensity projection of evoked activity-levels within the insular ROI in response to 8 different faces (columns) before (top three rows) and after conditioning (bottom three rows). 4th and 8th columns depict responses to CS+ and CS–, respectively. Note that the shape of the ROI matches the ones shown within the glass brain in (a). Color values represent beta-weights averaged across all subjects. Note the similarity of CS+ pattern with UCS pattern shown in Figure 4b (D: dorsal, L: left, R: right, A: anterior, P: posterior).
Supplementary Figure 8 Multivariate pattern similarity between activity evoked by different faces and UCS.
First panel: Same data as in Fig. 4d shown for comparison. Second, third and fourth panels depict similarity between activity patterns evoked by different faces and UCS for hippocampus, vmPFC and ITC, respectively. Error bars represent 95% bootstrap confidence intervals. Only in the aIC, a Gaussian model (black curve) performed significantly better than the null model (horizontal line, P < 0.005, likelihood-ratio test; vmPFC: ventromedial prefrontal cortex; ITC: inferotemporal cortex; aIC: anterior insula).
Supplementary Figure 9 Fear tuning in amygdala.
Temporal evolution of fear tuning in right amygdala during the test phase in [16.5 -7.5 -16.5] (xyz in mm). Fear tuning is shown for early (first panel), middle (second panel) and late temporal (third panel) windows. The x-axis represents responses to different faces. Responses to CS+ and CS– are shown at the 4th and 8th columns, respectively. Black curves and lines indicate best fitting models (Gaussian or null models; P <.01, likelihood-ratio test). The Gaussian model explained fear-tuning significantly better (P = 0.025, likelihood-ratio test) than the null model only in the early temporal window.
Supplementary Figure 10 Field of view during fMRI acquisition.
The field of view included the complete ventral part of the brain and was automatically co-registered across participants before the start of the scanning sessions to minimize loss of voxels due to lack of overlap.
Supplementary Figure 11 Hierarchical model used for parameter estimation.
Hierarchical dependencies between different parameters of the Bayesian model used for parameterization of fear generalization profiles. yi represents the ith observation recorded in response to ith face, which is xi radians distant from the CS+ face. The generalization profile of each single participant, p, within a given source, s (e.g. insula, or skin conductance responses), is modeled with αp,s and σp,s parameters of a Gaussian function, G. The error is assumed to be normally distributed (the “~” sign indicates stochastic relationships) with parameters μi and τs. τs is inverse of noise variance and specific for a given source, s. τs is half-Cauchy distributed (Gelman A., Bayesian Analysis 1, 515-534, 2006) with scale value of 5. μi is deterministically (the “=” sign indicates equalities) related to αp,s and σp,s parameters. In a given source s, αp is assumed to be distributed normally with parameters  and
and  , whereas the tuning-width parameter, σp, is assumed to originate from a log-normal distribution with parameters
, whereas the tuning-width parameter, σp, is assumed to originate from a log-normal distribution with parameters  and
and  .
.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Tables 1 and 2 (PDF 1675 kb)
Rights and permissions
About this article
Cite this article
Onat, S., Büchel, C. The neuronal basis of fear generalization in humans. Nat Neurosci 18, 1811–1818 (2015). https://doi.org/10.1038/nn.4166
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4166
This article is cited by
-
Chronic gut inflammation impairs contextual control of fear
Scientific Reports (2022)
-
Differential effects of the translocator protein 18 kDa (TSPO) ligand etifoxine and the benzodiazepine alprazolam on startle response to predictable threat in a NPU-threat task after acute and short-term treatment
Psychopharmacology (2022)
-
Associative learning and extinction of conditioned threat predictors across sensory modalities
Communications Biology (2021)
-
Intranasal oxytocin decreases fear generalization in males, but does not modulate discrimination threshold
Psychopharmacology (2021)
-
Neural correlates of conceptual-level fear generalization in posttraumatic stress disorder
Neuropsychopharmacology (2020)