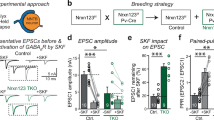
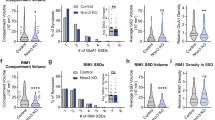
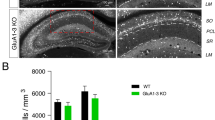
Abstract
α- and β-neurexins are presynaptic cell-adhesion molecules whose general importance for synaptic transmission is well documented. The specific functions of neurexins, however, remain largely unknown because no conditional neurexin knockouts are available and targeting all α- and β-neurexins produced by a particular gene is challenging. Using newly generated constitutive and conditional knockout mice that target all neurexin-3α and neurexin-3β isoforms, we found that neurexin-3 was differentially required for distinct synaptic functions in different brain regions. Specifically, we found that, in cultured neurons and acute slices of the hippocampus, extracellular sequences of presynaptic neurexin-3 mediated trans-synaptic regulation of postsynaptic AMPA receptors. In cultured neurons and acute slices of the olfactory bulb, however, intracellular sequences of presynaptic neurexin-3 were selectively required for GABA release. Thus, our data indicate that neurexin-3 performs distinct essential pre- or postsynaptic functions in different brain regions by distinct mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bang, M.L. & Owczarek, S. A matter of balance: role of neurexin and neuroligin at the synapse. Neurochem. Res. 38, 1174–1189 (2013).
Knight, D., Xie, W. & Boulianne, G.L. Neurexins and neuroligins: recent insights from invertebrates. Mol. Neurobiol. 44, 426–440 (2011).
Krueger, D.D., Tuffy, L.P., Papadopoulos, T. & Brose, N. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr. Opin. Neurobiol. 22, 412–422 (2012).
Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Tabuchi, K. & Südhof, T.C. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics 79, 849–859 (2002).
Ushkaryov, Y.A. et al. Conserved domain structure of beta-neurexins. Unusual cleaved signal sequences in receptor-like neuronal cell-surface proteins. J. Biol. Chem. 269, 11987–11992 (1994).
Ushkaryov, Y.A., Petrenko, A.G., Geppert, M. & Südhof, T.C. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science 257, 50–56 (1992).
Reichelt, A.C., Rodgers, R.J. & Clapcote, S.J. The role of neurexins in schizophrenia and autistic spectrum disorder. Neuropharmacology 62, 1519–1526 (2012).
Kim, H.G. et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am. J. Hum. Genet. 82, 199–207 (2008).
Kirov, G. et al. Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophr. Bull. 35, 851–854 (2009).
Sato, N. et al. Association between neurexin 1 (NRXN1) polymorphisms and the smoking behavior of elderly Japanese. Psychiatr. Genet. 20, 135–136 (2010).
Docampo, E. et al. Association of neurexin 3 polymorphisms with smoking behavior. Genes Brain Behav. 11, 704–711 (2012).
Hishimoto, A. et al. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum. Mol. Genet. 16, 2880–2891 (2007).
Kelai, S. et al. Nrxn3 upregulation in the globus pallidus of mice developing cocaine addiction. Neuroreport 19, 751–755 (2008).
Lachman, H.M. et al. Genome-wide suggestive linkage of opioid dependence to chromosome 14q. Hum. Mol. Genet. 16, 1327–1334 (2007).
Novak, G., Boukhadra, J., Shaikh, S.A., Kennedy, J.L. & Le Foll, B. Association of a polymorphism in the NRXN3 gene with the degree of smoking in schizophrenia: a preliminary study. World J. Biol. Psychiatry 10, 929–935 (2009).
Prats-Puig, A. et al. Variations in the obesity genes FTO, TMEM18 and NRXN3 influence the vulnerability of children to weight gain induced by short sleep duration. Int. J. Obes. (Lond.) 37, 182–187 (2013).
Treutlein, B., Gokce, O., Quake, S.R. & Südhof, T.C. Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proc. Natl. Acad. Sci. USA 111, E1291–E1299 (2014).
Ullrich, B., Ushkaryov, Y.A. & Südhof, T.C. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron 14, 497–507 (1995).
Aoto, J., Martinelli, D.C., Malenka, R.C., Tabuchi, K. & Südhof, T.C. Presynaptic Nrxn3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell 154, 75–88 (2013).
Missler, M. et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423, 939–948 (2003).
Chen, Y.C. et al. Drosophila neuroligin 2 is required presynaptically and postsynaptically for proper synaptic differentiation and synaptic transmission. J. Neurosci. 32, 16018–16030 (2012).
Hu, Z. et al. Neurexin and neuroligin mediate retrograde synaptic inhibition in C. elegans. Science 337, 980–984 (2012).
Fowler, S.C. et al. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J. Neurosci. Methods 107, 107–124 (2001).
Dobrunz, L.E. & Stevens, C.F. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18, 995–1008 (1997).
Hessler, N.A., Shirke, A.M. & Malinow, R. The probability of transmitter release at a mammalian central synapse. Nature 366, 569–572 (1993).
Murthy, V.N., Sejnowski, T.J. & Stevens, C.F. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron 18, 599–612 (1997).
Rosenmund, C., Clements, J.D. & Westbrook, G.L. Nonuniform probability of glutamate release at a hippocampal synapse. Science 262, 754–757 (1993).
Lin, J.W. et al. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat. Neurosci. 3, 1282–1290 (2000).
Cao, P., Maximov, A. & Südhof, T.C. Activity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10. Cell 145, 300–311 (2011).
Kaeser, P.S. et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144, 282–295 (2011).
Maximov, A., Pang, Z.P., Tervo, D.G. & Südhof, T.C. Monitoring synaptic transmission in primary neuronal cultures using local extracellular stimulation. J. Neurosci. Methods 161, 75–87 (2007).
Pang, Z.P. et al. Doc2 supports spontaneous synaptic transmission by a Ca(2+)-independent mechanism. Neuron 70, 244–251 (2011).
Zhang, C. et al. Neurexins physically and functionally interact with GABA(A) receptors. Neuron 66, 403–416 (2010).
Aoto, J., Nam, C.I., Poon, M.M., Ting, P. & Chen, L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron 60, 308–320 (2008).
Nam, C.I. & Chen, L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc. Natl. Acad. Sci. USA 102, 6137–6142 (2005).
Acknowledgements
We thank D. Martinelli, P. Rothwell, L. Chen and R.C. Malenka for advice and technical help. This study was supported by grants from the National Institute of Mental Health (R37MH52804 to T.C.S. and 1K99MH103531to J.A.), the National Institute on Drug Abuse (K99DA034029 to C.F.) and the American Heart Association, Western States (11POST7360078 to J.A.).
Author information
Authors and Affiliations
Contributions
J.A., K.T. and T.C.S. conceived the project. K.T. generated the Nrxn3α/β cKO mice and performed biochemical analyses of protein expression. J.A. designed and performed immunocytochemistry, culture and acute hippocampal slice electrophysiology, and animal behavior experiments. S.M.C.I. performed olfactory bulb immunocytochemistry. J.A. and C.F. performed olfactory bulb acute slice analyses. J.A. and T.C.S. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Protein expression levels are unchanged in constitutive Nrxn3α/β KO brains.
a. Representative Western blots - cropped images for 25 neuronal proteins in brain homogenates harvested from three sets of adult littermate wild-type and Nrxn3a/b KO mice.
b. Quantification of protein concentrations in adult wild-type and Nrxn3α/β KO brains (n=3 mice each).
c. qRT-PCR from mRNA isolated from wild-type, Nrxn3α+/– and Nrxn3α–/– brains and probed for transcripts representing Nrxn3α (left) and Nrxn3β (right). Note that based on the KO strategy (Fig. 1), the 5’ end of the Nrxn3α mRNA is still synthesized, but the resulting RNA is likely to nonsense-mediated decay resulting in a reduced level of mRNA for Nrxn3α. If the low levels of truncated mRNA produced a stable protein despite the presence of a partial C-terminal LNS-domain that is unable to fold due to the truncation, the produced protein would be secreted because it has no membrane anchor, and thus would be non-functional in all assays we have performed (see Fig. 7 this paper, and Aoto et al., 2013; Nrxn3α expression: Nrxn3α/β+/– vs. Nrxn3α/β –/–, p < 0.0001; Nrxn3β expression: Nrxn3α/β+/– vs. Nrxn3α/β –/–, p < 0.0001).
All data are shown as percent of control. Protein concentration levels were determined by phosphorimager analysis using I125 conjugated secondary antibodies. Data are means ± SEM. *, p<0.05 by Student’s t test comparing genotype protein levels and single factor ANOVA compared qRT-PCR.
Supplementary Figure 2 Comparison of Nrxn3α/β cKO and Nrxn3α KO on synaptic properties of cultured hippocampal neurons.
a. Conditional Nrxn3α/β KO does not alter passive membrane properties (left, input resistance; right, membrane capacitance). Hippocampal neurons were cultured from newborn homozygous Nrxn3α/β cKO mice, infected with inactive (control) or active cre-recombinase (cre) at DIV2-4, and analyzed on DIV14-16 (Rm: p = 0.612; Cm: p = 0.646).
b & c. mEPSC and mIPSC kinetics are not changed by the conditional Nrxn3α/β KO. Representative traces (top) and summary graphs (bottom) of mEPSC (b; rise: p = 0.995; decay: p = 0.861) and mIPSC (c; mIPSCs: rise: p = 0.876; decay: p = 0.800) rise and decay kinetics, measured in neurons obtained as in (a).
d. The Nrxn3α/β cKO does not decrease the release probability at excitatory synapses, measured using the stimulus-dependent NMDAR blockade by MK-801 during low-frequency stimulation. Neurons were obtained as described in a (p = 0.710).
e. Constitutive Nrxn3α KO does not alter passive membrane properties (left, input resistance; right, membrane capacitance). (Rm: p = 0.569; Cm: p = 0.670).
f. Constitutive Nrxn3α KO does not alter spontaneous mEPSCs (left, representative traces; right, summary graphs of the mEPSC frequency (p = 0.0676) and amplitude (p = 0.613)..
g-i. Constitutive Nrxn3α KO does not alter evoked AMPAR-mediated (g; p = 0.842) or NMDAR-mediated EPSCs (h; p = 0.767) or the PPR of NMDAR-mediated EPSCs (i; 100ms ISI: p = 0.553). For each panel, representative traces are shown on the left and summary graphs on the right..
Data shown represent means ± SEM of individual experiments; number of cells patched/experiment analyzed are shown in the bars or plots. For (i), mean ± SEM was calculated from number of cells. Statistical significance was assessed by single-factor ANOVA.
Supplementary Figure 3 Morphological quantification of synaptic parameters and GluA1 endocytosis in cultured hippocampal neurons.
a-c, Summary graphs representing the absolute puncta quantification of GluA1, GluA2 VGluT1 and PSD95 density (a; GluA1: p = 0.694; GluA2: p = 0.714; PSD95: p = 0.819; vGluT1: p = 0.758), size (b; GluA1: p = 0.0213; GluA2: p = 0.0424; PSD95: p = 0.188; vGluT1: p = 0.421) and intensity (c; GluA1: p = 0.579; GluA2: p = 0.267; vGluT1: p = 0.796) in control and cre infected Nrxn3α/βcKO neurons.
d, Summary graph representing levels of GluA1 internalization normalized to the control condition (p = 0.0110).
Data shown represent means ± SEM. Data were collected from 5 independent experiments. Statistical significance was assessed by single-factor ANOVA.
Supplementary Figure 4 Representative traces of neurexin rescue experiments.
a & b. Nrxn3α/β cKO neurons were co-infected with inactive (control) or active (cre) cre-recombinase at DIV 4 and with a virus that expressed (a) an endogenously expressed soluble isoform of Nrxn3SS4– (solNrxn3βSS–-) that results from alternative splicing in splice-site 5 and (b) the extracellular domain of neurexin-1βSS4– was fused to the transmembrane and intracellular domains of PDGFR (pDisNrxn1βSS4-). This construct permits trans-synaptic signaling while inhibiting intracellular signaling.
Supplementary Figure 5 Characterization of burst and regular firing subicular neurons.
a & b, Sample traces of intrinsic spiking properties used to identify burst (a) and regular (b) firing subicular projection neurons. Postsynaptic subicular neurons were recorded in whole cell current clamp mode and currents steps were injected.
c & d, Summary graphs representing the passive membrane properties of burst (c; input resistance: p = 0.719; capacitance: p = 0.796) and regular (d; input resistance: p = 0.696; capacitance: p = 0.929) firing cells. Measurements were recorded from uninfected postsynaptic subicular neurons in Nrxn3α/β cKO slices that had been stereotactically injected with AAVs expressing GFP fused to inactive (control) or active (cre) cre-recombinase in the CA1 region of the intermediate hippocampus.
Data shown represent means ± SEM calculated from number of cells. Numbers in bar graphs represent number of cells/independent experiments. Statistical significance was assessed by single-factor ANOVA.
Supplementary Figure 6 Characterization of excitatory synaptic transmission in Nrxn3 mutant olfactory bulb neurons.
a-c, Quantification of immunocytochemistry of Nrxn3α/β cKO olfactory bulb cultures infected with lentiviruses expressing inactive (control) or active (cre) cre-recombinase fused to EGFP. Puncta density (b; gephyrin: p = 0.884; vGAT: p = 0.176; vGluT1: p = 0.465), size (c; gephyrin: p = 0.262; vGAT: p = 0.170; vGluT1: p = 0.645) and intensity (d; gephyrin: p = 0.737; vGAT: p = 0.851; vGluT1: p = 0.693) representing gephyrin, vGAT and vGluT1 immunoreactivity.
d, Summary graphs of the passive membrane properties measured in mitral/tufted cells in Nrxn3α/β cKO olfactory bulb cultures infected with lentiviruses expressing inactive (control) or active (cre) cre-recombinase fused to EGFP (input resistance: p = 0.728; capacitance: p = 0.583).
e, Sample traces (left) and summary graphs representing mEPSC kinetics measured in Nrxn3α/β cKO olfactory bulb cultures infected with lentiviruses expressing inactive (control) or active (cre) cre-recombinase fused to EGFP (mEPSC rise: p = 0.816; decay: p = 0.286).
f, Same as in (d) but passive membrane properties measured in mitral/tufted cells from Nrxn3SS4+ KI olfactory bulb cultures infected with lentiviruses expressing inactive (Nrxn3SS4+) or active (Nrxn3SS4–) cre-recombinase fused to EGFP (input resistance: p = 0.921; capacitance: 0.894).
g, mEPSC kinetics from Nrxn3SS4+ or Nrxn3SS4– primary olfactory bulb cultures (mEPSC rise: p = 0.626; decay: p = 0.693).
Data shown represent means ± SEM calculated from total number of experiments. Numbers in bar graphs represent number of cells/independent experiments. Statistical significance was assessed by single-factor ANOVA.
Supplementary Figure 7 Properties of mIPSCs in cultured olfactory bulb neurons from the three different types Nrxn3 mutant animals.
a. mIPSC cumulative probability plots for frequency (left) and amplitude (right) from Nrxn3α/β cKO mitral/tufted neurons in (p < 0.0001).
b, Representative traces (left) and summary graphs (right) of mIPSC decay and rise kinetics from Nrxn3α/β cKO mitral/tufted neurons in (mIPSC rise: p = 0.462; decay: p = 0.991).
c, Representative traces (left) and summary graph (right) measuring the sucrose-evoked readily releasable pool (RRP) of GABAergic vesicles in Nrxn3α/β cKO olfactory bulb cultures (p = 0.303).
d & f, mIPSC cumulative probability plots as in (a) but from cultured Nrxn3α KO mitral/tufted neurons (d; p < 0.0001) or from cultured Nrxn3 SS#4 KI neurons infected with lentiviruses expressing inactive Cre (Nrxn3SS4+) or active Cre (Nrxn3SS4-) recombinase (f; p = 0.671).
e & g, mIPSC kinetics as in (b) but in Nrxn3α KO mitral/tufted neurons (e; mIPSC rise: p = 0.939; decay: p = 0.943) and Nrxn3SS#4 KI (g; mIPSC rise: p = 0.520; decay: p = 0.713).
h & i, Cartoon schematic of full-length (h) and GPI-anchored (i) Nrxn3α rescue constructs used in Figs. 7d and 7e. Note: GPI-Nrxn3α is incapable of activating intracellular signaling.
Data represent means ± SEM calculated from independent experiments. Numbers in bar graphs represent number of cells/independent experiments. Statistical significance in b, c, e, g was assessed by single-factor ANOVA. Statistical significance for cumulative probability plots was determined by K-S test.
Supplementary Figure 8 Dendrodendritic synapse diagram and confirmation of proper AAV targeting.
a, Cartoon model of a dendrodendritic granule cell-mitral cell synapse. Antidromc stimulation of mitral cell axons in the lateral olfactory tract evoke the dendritic release of glutamate onto a granule cell spine. Ca2+ influx via NMDARs located in the granule cell spine cause the release of GABA onto the mitral cell dendrite.
b, Representative coronal section of a Nrxn3α/β cKO olfactory bulb stereotactically injected with AAVs expressing GFP fused to inactive or active cre-recombinase. Note the complete infection (GFP+) of the granule cell layer.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 1034 kb)
Source data
Rights and permissions
About this article
Cite this article
Aoto, J., Földy, C., Ilcus, S. et al. Distinct circuit-dependent functions of presynaptic neurexin-3 at GABAergic and glutamatergic synapses. Nat Neurosci 18, 997–1007 (2015). https://doi.org/10.1038/nn.4037
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4037
This article is cited by
-
A combinatorial code of neurexin-3 alternative splicing controls inhibitory synapses via a trans-synaptic dystroglycan signaling loop
Nature Communications (2023)
-
Distinct neurexin isoforms cooperate to initiate and maintain foraging activity
Translational Psychiatry (2023)
-
Neurexin-3 subsynaptic densities are spatially distinct from Neurexin-1 and essential for excitatory synapse nanoscale organization in the hippocampus
Nature Communications (2023)
-
Conditional deletion of Neurexin-2 alters neuronal network activity in hippocampal circuitries and leads to spontaneous seizures
Translational Psychiatry (2023)
-
Shank2/3 double knockout-based screening of cortical subregions links the retrosplenial area to the loss of social memory in autism spectrum disorders
Molecular Psychiatry (2022)