Abstract
Diverse types of local GABAergic interneurons shape the cortical representation of sensory information. Here we show how somatostatin-expressing interneurons (SOM cells) contribute to odor coding in mouse olfactory cortex. We find that odor-tuned SOM cells regulate principal cells through a purely subtractive operation that is independent of odor identity or intensity. This operation enhances the salience of odor-evoked activity without changing cortical odor tuning. SOM cells inhibit both principal cells and fast-spiking interneurons, indicating that subtractive inhibition reflects the interplay of multiple classes of interneurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Silver, R.A. Neuronal arithmetic. Nat. Rev. Neurosci. 11, 474–489 (2010).
Isaacson, J.S. & Scanziani, M. How inhibition shapes cortical activity. Neuron 72, 231–243 (2011).
Wilson, N.R., Runyan, C.A., Wang, F.L. & Sur, M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348 (2012).
Lee, S.-H. et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488, 379–383 (2012).
Atallah, B.V., Bruns, W., Carandini, M. & Scanziani, M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73, 159–170 (2012).
Cottam, J.C.H., Smith, S.L. & Häusser, M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J. Neurosci. 33, 19567–19578 (2013).
Lee, S.-H., Kwan, A.C. & Dan, Y. Interneuron subtypes and orientation tuning. Nature 508, E1–E2 (2014).
Atallah, B.V., Scanziani, M. & Carandini, M. & Atallah et al. reply. Nature 508, E3 (2014).
El-Boustani, S., Wilson, N.R., Runyan, C.A. & Sur, M. & El-Boustani et al. reply. Nature 508, E3–E4 (2014).
Wilson, D.A. & Sullivan, R.M. Cortical processing of odor objects. Neuron 72, 506–519 (2011).
Suzuki, N. & Bekkers, J.M. Inhibitory neurons in the anterior piriform cortex of the mouse: classification using molecular markers. J. Comp. Neurol. 518, 1670–1687 (2010).
Suzuki, N. & Bekkers, J.M. Distinctive classes of GABAergic interneurons provide layer-specific phasic inhibition in the anterior piriform cortex. Cereb. Cortex 20, 2971–2984 (2010).
Chow, B.Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Boyd, A.M., Sturgill, J.F., Poo, C. & Isaacson, J.S. Cortical feedback control of olfactory bulb circuits. Neuron 76, 1161–1174 (2012).
Royer, S. et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 15, 769–775 (2012).
Poo, C. & Isaacson, J.S. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron 62, 850–861 (2009).
Tolhurst, D.J., Movshon, J.A. & Dean, A.F. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res. 23, 775–785 (1983).
Duguid, I., Branco, T., London, M., Chadderton, P. & Häusser, M. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J. Neurosci. 32, 11132–11143 (2012).
Pfeffer, C.K., Xue, M., He, M., Huang, Z.J. & Scanziani, M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 16, 1068–1076 (2013).
Stokes, C.C.A. & Isaacson, J.S. From dendrite to soma: dynamic routing of inhibition by complementary interneuron microcircuits in olfactory cortex. Neuron 67, 452–465 (2010).
Suzuki, N. & Bekkers, J.M. Microcircuits mediating feedforward and feedback synaptic inhibition in the piriform cortex. J. Neurosci. 32, 919–931 (2012).
Adesnik, H., Bruns, W., Taniguchi, H., Huang, Z.J. & Scanziani, M. A neural circuit for spatial summation in visual cortex. Nature 490, 226–231 (2012).
Xu, H., Jeong, H.-Y., Tremblay, R. & Rudy, B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron 77, 155–167 (2013).
Stettler, D.D. & Axel, R. Representations of odor in the piriform cortex. Neuron 63, 854–864 (2009).
Bortone, D.S., Olsen, S.R. & Scanziani, M. Translaminar inhibitory cells recruited by layer 6 corticothalamic neurons suppress visual cortex. Neuron 82, 474–485 (2014).
Acknowledgements
We thank M. Scanziani, K. Franks, P. Frady and members of the Isaacson and Scanziani laboratories for advice and discussions. Supported by the US National Institute on Deafness and Other Communication Disorders (R01DC04682, J.S.I.; 5F32DC013511, J.F.S.).
Author information
Authors and Affiliations
Contributions
J.F.S. and J.S.I. performed experiments, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
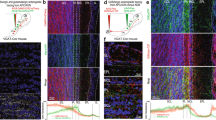
Supplementary Figure 1 Expression of Arch-GFP in somatostatin-expressing interneurons.
Top, GFP fluorescence (green) in a coronal section of anterior piriform cortex from a SOM-cre mouse injected with AAV-2/9-CBA-Flexed-Arch-GFP. Middle, somatostatin immunofluorescence (red) from the same section. Bottom, merge of red and green channels. Left, low power images reveal robust expression of Arch-GFP and immunolabeled cell bodies limited to layer 3. Right, higher magnification image illustrating Arch-expressing somata immunopositive for somatostatin (overlap=79% ± 4%, n=104 cells from 3 mice).
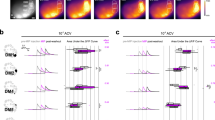
Supplementary Figure 2 Properties of optogenetically tagged SOM cell units.
a) Histogram of LED modulation index (see Methods) for all single units. Red, putative SOM cell units (n=11); blue, non-SOM cell units (n=101). b) Odor intensity-response relationship of SOM cell units (n=7) shows consistent LED-induced suppression of SOM cell activity across different odor intensities. c) Odor tuning of SOM cell units (red, n=4) is similar to tuning of odor-activated principal cells (blue, n=18). d) Summary of firing activity for individual SOM cell units tested with different odors and odor intensities (grey lines, linear fits to individual units). Thick black line is average linear fit across all SOM cell units (n=11) shows a slope (m) of 0.2 (p<0.005) indicating a consistent LED-induced reduction in SOM cell activity.
Supplementary Figure 3 Effect of SOM cell suppression on layer 2/3 single-unit odor intensity–response relationship.
a) PSTHs from representative unit with and without LED illumination.b) Linear fit to the data shown in A reveals shift in y intercept (b) while slope (m) remains near unity. c) Summary of results from 21 units. Grey circles and grey lines; responses and linear fits to individual cells. Thick black line, average linear fit indicates additive shift in activity in response to SOM cell suppression.
Supplementary Figure 4 ChR2 activation of PV cells causes the divisive scaling of single-unit firing rates in piriform cortex.
a) Raster plots and trial histograms for a representative unit during application of different concentrations of amyl acetate under control (black) and PV cell activation (red) conditions. b) Linear fit indicating a significant reduction in slope of the relationship between LED off and LED on firing rates across odor intensity of the example unit in A. c) Summary of 4 experiments and 21 units (grey circles and grey lines) and average linear fit (thick black line) demonstrating that PV cell activation causes a linear offset and divisive scaling of firing rates in PCx (slope=0.46±0.08, p<0.0005, y-intercept=-0.34±0.24, p<0.001, n=21 units).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 728 kb)
Rights and permissions
About this article
Cite this article
Sturgill, J., Isaacson, J. Somatostatin cells regulate sensory response fidelity via subtractive inhibition in olfactory cortex. Nat Neurosci 18, 531–535 (2015). https://doi.org/10.1038/nn.3971
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3971
This article is cited by
-
Intrinsic plasticity of Purkinje cell serves homeostatic regulation of fear memory
Molecular Psychiatry (2023)
-
Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders
Molecular Psychiatry (2021)
-
Associative conditioning remaps odor representations and modifies inhibition in a higher olfactory brain area
Nature Neuroscience (2019)
-
Spatial suppression promotes rapid figure-ground segmentation of moving objects
Nature Communications (2019)
-
Spatially segregated feedforward and feedback neurons support differential odor processing in the lateral entorhinal cortex
Nature Neuroscience (2016)