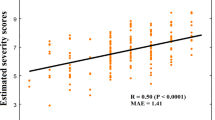
Abstract
Autism spectrum disorder (ASD) has been associated with a reduction in resting state functional connectivity, though this assertion has recently been challenged by reports of increased connectivity in ASD. To address these contradictory findings, we examined both inter- and intrahemispheric functional connectivity in several resting state data sets acquired from adults with high-functioning ASD and matched control participants. Our results reveal areas of both increased and decreased connectivity in multiple ASD groups as compared to control groups. We propose that this heterogeneity stems from a previously unrecognized ASD characteristic: idiosyncratic distortions of the functional connectivity pattern relative to the typical, canonical template. The magnitude of an individual's pattern distortion in homotopic interhemispheric connectivity correlated significantly with behavioral symptoms of ASD. We propose that individualized alterations in functional connectivity organization are a core characteristic of high-functioning ASD, and that this may account for previous discrepant findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Biswal, B., Yetkin, F.Z., Haughton, V.M. & Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 (1995).
Raichle, M.E. et al. A default mode of brain function. Proc. Natl. Acad. Sci. USA 98, 676–682 (2001).
Salvador, R. et al. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb. Cortex 15, 1332–1342 (2005).
Nir, Y. et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat. Neurosci. 11, 1100–1108 (2008).
He, B.J., Snyder, A.Z., Zempel, J.M., Smyth, M.D. & Raichle, M.E. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc. Natl. Acad. Sci. USA 105, 16039–16044 (2008).
Stark, D.E. et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J. Neurosci. 28, 13754–13764 (2008).
Buckner, R.L., Andrews-Hanna, J.R. & Schacter, D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. NY Acad. Sci. 1124, 1–38 (2008).
American Psychiatric Association. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Press, Washington, DC, 2000).
Just, M.A., Cherkassky, V.L., Keller, T.A. & Minshew, N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 127, 1811–1821 (2004).
Belmonte, M.K. et al. Autism and abnormal development of brain connectivity. J. Neurosci. 24, 9228–9231 (2004).
Schipul, S.E., Keller, T.A. & Just, M.A. Inter-regional brain communication and its disturbance in autism. Front. Syst. Neurosci. 5, 10 (2011).
Dinstein, I. et al. Disrupted neural synchronization in toddlers with autism. Neuron 70, 1218–1225 (2011).
Anderson, J.S. et al. Decreased interhemispheric functional connectivity in autism. Cereb. Cortex 21, 1134–1146 (2011).
Di Martino, A. et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 19, 659–667 (2014).
Keown, C.L. et al. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Reports 5, 567–572 (2013).
Supekar, K. et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Reports 5, 738–747 (2013).
Müller, R.A. et al. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb. Cortex 21, 2233–2243 (2011).
Uddin, L.Q. et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry 70, 869–879 (2013).
Lynch, C.J. et al. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol. Psychiatry 74, 212–219 (2013).
Tibshirani, R., Walther, G. & Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Series B Stat. Methodol. 63, 411–423 (2001).
Belmonte, M.K., Gomot, M. & Baron-Cohen, S. Visual attention in autism families: ‘unaffected’ sibs share atypical frontal activation. J. Child Psychol. Psychiatry 51, 259–276 (2010).
Lord, C. et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 30, 205–223 (2000).
Lord, C., Rutter, M. & Le Couteur, A. Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 24, 659–685 (1994).
Anagnostou, E. & Taylor, M.J. Review of neuroimaging in autism spectrum disorders: what have we learned and where we go from here. Mol. Autism 2, 4 (2011).
Tyszka, J.M., Kennedy, D.P., Paul, L.K. & Adolphs, R. Largely typical patterns of resting-state functional connectivity in high-functioning adults with autism. Cereb. Cortex 24, 1894–1905 (2014).
Carper, R.A. & Courchesne, E. Localized enlargement of the frontal cortex in early autism. Biol. Psychiatry 57, 126–133 (2005).
Müller, R.A., Pierce, K., Ambrose, J.B., Allen, G. & Courchesne, E. Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biol. Psychiatry 49, 665–676 (2001).
Müller, R.A., Kleinhans, N., Kemmotsu, N., Pierce, K. & Courchesne, E. Abnormal variability and distribution of functional maps in autism: an FMRI study of visuomotor learning. Am. J. Psychiatry 160, 1847–1862 (2003).
Scherf, K.S., Luna, B., Minshew, N. & Behrmann, M. Location, location, location: alterations in the functional topography of face- but not object- or place-related cortex in adolescents with autism. Front. Hum. Neurosci. 4, 26 (2010).
Pierce, K., Muller, R.A., Ambrose, J., Allen, G. & Courchesne, E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain 124, 2059–2073 (2001).
Hasson, U. et al. Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Res. 2, 220–231 (2009).
Hahamy, A. et al. Normalisation of brain connectivity through compensatory behaviour, despite congenital hand absence. eLife 4, e4605 (2015).
Harmelech, T. & Malach, R. Neurocognitive biases and the patterns of spontaneous correlations in the human cortex. Trends Cogn. Sci. 17, 606–615 (2013).
Sadaghiani, S. & Kleinschmidt, A. Functional interactions between intrinsic brain activity and behavior. Neuroimage 80, 379–386 (2013).
Dinstein, I. et al. Unreliable evoked responses in autism. Neuron 75, 981–991 (2012).
Markram, H., Rinaldi, T. & Markram, K. The intense world syndrome–an alternative hypothesis for autism. Front Neurosci 1, 77–96 (2007).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 (2002).
Greve, D.N. & Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72 (2009).
Zhang, Y., Brady, M. & Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 20, 45–57 (2001).
Power, J.D., Barnes, K.A., Snyder, A.Z., Schlaggar, B.L. & Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Hallquist, M.N., Hwang, K. & Luna, B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 82, 208–225 (2013).
Fox, M.D., Zhang, D., Snyder, A.Z. & Raichle, M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 101, 3270–3283 (2009).
Hahamy, A. et al. Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect. 4, 395–403 (2014).
Saad, Z.S. et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2, 25–32 (2012).
Fisher, R.A. Statistical Methods for Research Workers (Genesis Publishing, 1925).
Fisher, R. Questions and answers #14. Am. Stat. 2, 30–31 (1948).
Stouffer, S.A., Suchman, E.A., DeVinney, L.C., Star, S.A. & Williams, R.M. Jr. The American Soldier, Vol. 1: Adjustment during Army Life (Princeton Univ. Press, 1949).
Liptak, T. On the combination of independent tests. Magyar Tud. Akad. Mat. Kutato Int. Kozl. 3, 171–197 (1958).
Glover, G.H. et al. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J. Magn. Reson. Imaging 36, 39–54 (2012).
Hedges, L.V. & Olkin, I. Statistical Methods for Meta-analysis (Academic, Boston and London, 1985).
Turner, H.M. & Bernard, R.M. Calculating and synthesizing effect sizes. Contemp. Issues Commun. Sci. Disord. 33, 42–45 (2006).
Hentschke, H. & Stüttgen, M.C. Computation of measures of effect size for neuroscience data sets. Eur. J. Neurosci. 34, 1887–1894 (2011).
Douaud, G. et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130, 2375–2386 (2007).
Good, C.D. et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14, 21–36 (2001).
Smith, S.M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 (suppl. 1): S208–S219 (2004).
Acknowledgements
The authors would like to thank H. Dubossarsky and T. Golan for numerous discussions and suggestions that contributed greatly to this work, and Y. Cohen for assistance with illustrations. This study was supported by the Israeli Presidential Bursary for outstanding PhD students in brain research (A.H.); the Simons Foundation SFARI award 298640 and the Pennsylvania Department of Health SAP grant 4100047862 (M.B.); and a European Union Future Emerging Technologies -7 – Virtual Embodiment and Robotic Re-Embodiment, Israeli Science Foundation, and Israeli Center of Research Excellence grant 51/11, European Union Flagship The Human Brain Project and the Helen and Martin Kimmel award (R.M.). Funding sources for the ABIDE data sets are listed at http://fcon_1000.projects.nitrc.org/indi/abide/index.html.
Author information
Authors and Affiliations
Contributions
A.H. conducted the data analyses; A.H., M.B. and R.M. interpreted the results and wrote the manuscript; R.M. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Comparison of typical interhemispheric connectivity and group differences in interhemispheric connectivity by data set
(a) The bottom map of each column is the unthresholded between-group inter-hemispheric t-test map for each specific dataset (control>ASD). The top map of each column is the group inter-hemispheric connectivity map of control participants from all datasets, excluding participants of the specific dataset for which the t-map is presented below. The number of participants contained in each dataset is denoted in the title of each column. Arrows demonstrate the correspondence between directionalities of group-differences and variation in homotopic inter-hemispheric connectivity magnitudes; the ASD groups show reduced homotopic inter-hemispheric connectivity in regions of typically high inter-hemispheric connectivity (orange arrows), and increased inter-hemispheric connectivity in areas of reduced connectivity in the typical brain (blue arrows). Note the spatial similarity between disparity directionalities across datasets. (b) Top: Homotopic inter-hemispheric group map of all control and ASD participants combined across datasets. Bottom: Unthresholded pooled between-group t-test map (controls>ASD). Since homotopic inter-hemispheric maps are symmetrical across the midline, only the right hemisphere is presented. CAL, California Institute of Technology; CMU, Carnegie Mellon University; PBG, University of Pittsburgh; Utah-1, University of Utah – first half; Utah-2, University of Utah – second half; LOC, lateral occipital cortex; ITG, inferior temporal gyrus; PCG, post central gyrus; MFG, middle frontal gyrus.
Supplementary Figure 2 Pooled analysis versus meta-analysis of homotopic interhemispheric connectivity group differences
Left: t-test map (control>ASD) created for the pooled cohort of participants. Right: Meta-analysis map showing effect sizes (control>ASD) measured using Hedges' g. White contours represent areas found to show a significant difference between groups using 95% confidence intervals. Note the spatial similarity between the two maps in areas showing either over- or under-connectivity in ASD participants in comparison to controls.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 and Supplementary Tables 1–10 (PDF 1254 kb)
Rights and permissions
About this article
Cite this article
Hahamy, A., Behrmann, M. & Malach, R. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat Neurosci 18, 302–309 (2015). https://doi.org/10.1038/nn.3919
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3919
This article is cited by
-
Neurophysiological measures of auditory sensory processing are associated with adaptive behavior in children with Autism Spectrum Disorder
Journal of Neurodevelopmental Disorders (2023)
-
Diverging asymmetry of intrinsic functional organization in autism
Molecular Psychiatry (2023)
-
Endogenous noise of neocortical neurons correlates with atypical sensory response variability in the Fmr1−/y mouse model of autism
Nature Communications (2023)
-
Atypical connectivity aids conversation in autism
Scientific Reports (2023)
-
Sounds Pleasantness Ratings in Autism: Interaction Between Social Information and Acoustical Noise Level
Journal of Autism and Developmental Disorders (2023)