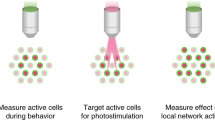
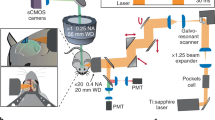
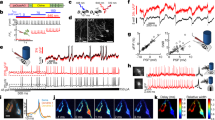
Abstract
Linking neural microcircuit function to emergent properties of the mammalian brain requires fine-scale manipulation and measurement of neural activity during behavior, where each neuron's coding and dynamics can be characterized. We developed an optical method for simultaneous cellular-resolution stimulation and large-scale recording of neuronal activity in behaving mice. Dual-wavelength two-photon excitation allowed largely independent functional imaging with a green fluorescent calcium sensor (GCaMP3, λ = 920 ± 6 nm) and single-neuron photostimulation with a red-shifted optogenetic probe (C1V1, λ = 1,064 ± 6 nm) in neurons coexpressing the two proteins. We manipulated task-modulated activity in individual hippocampal CA1 place cells during spatial navigation in a virtual reality environment, mimicking natural place-field activity, or 'biasing', to reveal subthreshold dynamics. Notably, manipulating single place-cell activity also affected activity in small groups of other place cells that were active around the same time in the task, suggesting a functional role for local place cell interactions in shaping firing fields.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Greenberg, D.S., Houweling, A.R. & Kerr, J.N. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat. Neurosci. 11, 749–751 (2008).
Dombeck, D.A., Harvey, C.D., Tian, L., Looger, L.L. & Tank, D.W. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 13, 1433–1440 (2010).
Petreanu, L. et al. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature 489, 299–303 (2012).
Adamantidis, A.R., Zhang, F., Aravanis, A.M., Deisseroth, K. & de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424 (2007).
Tye, K.M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Carter, M.E. et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533 (2010).
Akerboom, J. et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 6, 2 (2013).
Chang, Y.F., Arai, Y. & Nagai, T. Optogenetic activation during detector “dead time” enables compatible real-time fluorescence imaging. Neurosci. Res. 73, 341–347 (2012).
Husson, S.J. et al. Optogenetic analysis of a nociceptor neuron and network reveals ion channels acting downstream of primary sensors. Curr. Biol. 22, 743–752 (2012).
Lin, J.Y., Lin, M.Z., Steinbach, P. & Tsien, R.Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814 (2009).
Wilson, N.R., Runyan, C.A., Wang, F.L. & Sur, M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348 (2012).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007).
Guo, Z.V., Hart, A.C. & Ramanathan, S. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat. Methods 6, 891–896 (2009).
Rickgauer, J.P. & Tank, D.W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl. Acad. Sci. USA 106, 15025–15030 (2009).
Andrasfalvy, B.K., Zemelman, B.V., Tang, J. & Vaziri, A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc. Natl. Acad. Sci. USA 107, 11981–11986 (2010).
Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854 (2010).
Prakash, R. et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods 9, 1171–1179 (2012).
Packer, A.M. et al. Two-photon optogenetics of dendritic spines and neural circuits. Nat. Methods 9, 1202–1205 (2012).
Yaroslavsky, A.N. et al. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys. Med. Biol. 47, 2059–2073 (2002).
Oheim, M., Beaurepaire, E., Chaigneau, E., Mertz, J. & Charpak, S. Two-photon microscopy in brain tissue: parameters influencing the imaging depth. J. Neurosci. Methods 111, 29–37 (2001).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 (2009).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Oron, D., Tal, E. & Silberberg, Y. Scanningless depth-resolved microscopy. Opt. Express 13, 1468–1476 (2005).
Zhu, G., van Howe, J., Durst, M., Zipfel, W. & Xu, C. Simultaneous spatial and temporal focusing of femtosecond pulses. Opt. Express 13, 2153–2159 (2005).
Zimmermann, T., Rietdorf, J. & Pepperkok, R. Spectral imaging and its applications in live cell microscopy. FEBS Lett. 546, 87–92 (2003).
Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172 (2012).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2–assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668 (2007).
Aronov, D. & Tank, D.W. Engagement of the neural circuits underlying 2D spatial navigation in a rodent virtual reality system. Neuron 84, 442–456 (2014).
Harvey, C.D., Collman, F., Dombeck, D.A. & Tank, D.W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946 (2009).
Lee, D., Lin, B.J. & Lee, A.K. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science 337, 849–853 (2012).
Fenton, A.A. & Muller, R.U. Place cell discharge is extremely variable during individual passes of the rat through the firing field. Proc. Natl. Acad. Sci. USA 95, 3182–3187 (1998).
Ziv, Y. et al. Long-term dynamics of CA1 hippocampal place codes. Nat. Neurosci. 16, 264–266 (2013).
Kaifosh, P., Lovett-Barron, M., Turi, G.F., Reardon, T.R. & Losonczy, A. Septo-hippocampal GABAergic signaling across multiple modalities in awake mice. Nat. Neurosci. 16, 1182–1184 (2013).
Lovett-Barron, M. et al. Dendritic inhibition in the hippocampus supports fear learning. Science 343, 857–863 (2014).
Chen, T.W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
St-Pierre, F. et al. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat. Neurosci. 17, 884–889 (2014).
Grienberger, C., Chen, X. & Konnerth, A. NMDA receptor-dependent multidendrite Ca2+ spikes required for hippocampal burst firing in vivo. Neuron 81, 1274–1281 (2014).
Papagiakoumou, E. et al. Functional patterned multiphoton excitation deep inside scattering tissue. Nat. Photonics 7, 274–278 (2013).
Losonczy, A., Zemelman, B.V., Vaziri, A. & Magee, J.C. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 13, 967–972 (2010).
Dana, H. et al. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS ONE 9, e108697 (2014).
Amaral, D.G. & Witter, M.P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31, 571–591 (1989).
Deuchars, J. & Thomson, A.M. CA1 pyramid-pyramid connections in rat hippocampus in vitro: dual intracellular recordings with biocytin filling. Neuroscience 74, 1009–1018 (1996).
Marshall, L. et al. Hippocampal pyramidal cell-interneuron spike transmission is frequency dependent and responsible for place modulation of interneuron discharge. J. Neurosci. 22, RC197 (2002).
Galarreta, M. & Hestrin, S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science 292, 2295–2299 (2001).
Jonas, P., Bischofberger, J., Fricker, D. & Miles, R. Interneuron diversity series: fast in, fast out–temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 27, 30–40 (2004).
Freund, T.F. & Buzsaki, G. Interneurons of the hippocampus. Hippocampus 6, 347–470 (1996).
Ko, H. et al. Functional specificity of local synaptic connections in neocortical networks. Nature 473, 87–91 (2011).
Royer, S. et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 15, 769–775 (2012).
Briggman, K.L., Helmstaedter, M. & Denk, W. Wiring specificity in the direction-selectivity circuit of the retina. Nature 471, 183–188 (2011).
Vaziri, A. & Emiliani, V. Reshaping the optical dimension in optogenetics. Curr. Opin. Neurobiol. 22, 128–137 (2012).
Dana, H. & Shoham, S. Numerical evaluation of temporal focusing characteristics in transparent and scattering media. Opt. Express 19, 4937–4948 (2011).
Grewe, B.F., Voigt, F.F., van't Hoff, M. & Helmchen, F. Fast two-layer two-photon imaging of neuronal cell populations using an electrically tunable lens. Biomed. Opt. Express 2, 2035–2046 (2011).
Pologruto, T.A., Sabatini, B.L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Domnisoru, C., Kinkhabwala, A.A. & Tank, D.W. Membrane potential dynamics of grid cells. Nature 495, 199–204 (2013).
Harvey, C.D., Coen, P. & Tank, D.W. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484, 62–68 (2012).
Zimmermann, T., Rietdorf, J., Girod, A., Georget, V. & Pepperkok, R. Spectral imaging and linear un-mixing enables improved FRET efficiency with a novel GFP2-YFP FRET pair. FEBS Lett. 531, 245–249 (2002).
Miri, A., Daie, K., Burdine, R.D., Aksay, E. & Tank, D.W. Regression-based identification of behavior-encoding neurons during large-scale optical imaging of neural activity at cellular resolution. J. Neurophysiol. 105, 964–980 (2011).
Mukamel, E.A., Nimmerjahn, A. & Schnitzer, M.J. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron 63, 747–760 (2009).
Dombeck, D.A., Graziano, M.S. & Tank, D.W. Functional clustering of neurons in motor cortex determined by cellular resolution imaging in awake behaving mice. J. Neurosci. 29, 13751–13760 (2009).
Acknowledgements
We thank D. Kim and C. Guo (Genetically Encoded Neuronal Indicator and Effector Project, Janelia Research Campus) for transgenic mice, D. Aronov for VR software, B. Scott for discussions, and C. Domnisoru, A. Miri, F. Collman and S. Wang for comments on the manuscript. This work was supported by the US National Institutes of Health (R01-MH083686; P50-GM071508) and a National Science Foundation Graduate Research Fellowship to J.P.R.
Author information
Authors and Affiliations
Contributions
J.P.R. and D.W.T. designed the study. K.D. contributed reagents. J.P.R. and D.W.T. performed the experiments. J.P.R. analyzed data with strategy and methods contributions from D.W.T. J.P.R. and D.W.T. wrote the paper with comments from K.D.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Spectral properties of molecules, laser sources, and optics used in this approach.
a. Visible-wavelength regime. Shown are: fluorescence emission spectra for EGFP and EYFP (obtained from the Tsien Lab website, University of California, San Diego); the single-photon excitation spectrum for C1V1(E122T/E162T) (adapted from Yizhar et al., Nature 477, 171-178 [2011]); transmission curves for the dichroic (dark line) and emission filters (shaded areas) used in two-channel fluorescence detection (filter part numbers indicated); and the transmission curve for the long-pass laser-blocking filter (blue line; curves from Semrock). The 473 nm laser line used in single-photon excitation experiments is also indicated (dashed blue line). Each curve is normalized to its own peak value. b. Infrared-wavelength regime. Two-photon action cross section for GCaMP3 (green) and relative C1V1 photocurrent response amplitudes (see inset) sampled at two infrared TPE center wavelengths (λ=900 nm and λ=1050 nm). Inset: sample intracellular photocurrents from illuminated HEK293T cells expressing C1V1; peak squared-intensity values were similar (2.76x1054 γ2/cm4-s2 and 1.68x1054 γ2/cm4-s2 at 900 nm and 1050 nm; assuming a fixed output temporal pulse-width). The GCaMP3 action cross-section was measured using fluorescence excited by focused low-power illumination (regime of quadratic power dependence) of a purified GCaMP3.3 sample (37 μM concentration in 20 mM MOPS, 100 mM KCl, 2.7 mM K2CaEGTA, at pH 7.4; R. Sun and S. S.-H. Wang, Princeton), normalized at each wavelength using side-by-side measurements of a reference fluorophore (20 µM fluorescein in water, pH 11; see Albota, M. A., Xu, C. & Webb, W., Appl. Opt. 37, 7352–7356 [1998]). C1V1 wavelength-sensitivity was evaluated at two spectral bands (λ=900 nm and 1050 nm), using whole-cell electrode recordings at constant voltage (-50 mV) in HEK293T cells transiently expressing the pLenti-CaMKIIa-C1V1(E162T)-TS-EYFP construct with focused scanning methods and an apparatus described previously (Rickgauer and Tank, PNAS 106, 15025-15030 [2009]).
Supplementary Figure 2 Schematic for either single-photon or two-photon excitation (TPE) photostimulation and TPE imaging.
a. Position of optics used to introduce the SPE source into the TPE microscope head. Abbreviations: AM, alignment mirror; FT, focusing telescope; SP, short-pass filter; DC, dichroic filter; LP, long-pass filter; PMTs, photomultiplier tubes. b. TPE images (acquired at 920 nm) of a volume in a fluorescent plastic slide after bleaching neighboring areas using SPE (473 nm) and TPE (1064 nm, spatial focusing path; SF in Fig. 1, main text). Image intensity is inverted. Images are shown at the 1064 nm focal plane (upper) and as an xz projection of a through-focus series (lower).
Supplementary Figure 3 TPE stimulation evokes GCaMP3 transients consistent with action potentials (APs) and opsin-mediated depolarization in awake mice.
a. GCaMP3 ΔF/F values vs. stimulation pulse number. Somatic ΔF/F values for 6 neurons stimulated at 5, 10, and 20 Hz (16 ms per pulse), measured 500 ms after pulse-train onset (values for each cell are normalized to the peak response; average of 3-7 trials per data point). The monotonic relationship between ΔF/F and stimulation pulse number is consistent with a regime in which ΔF/F values also scale approximately linearly with AP number (assuming 1 AP per pulse; Tian et al., Nat. Methods 6, 875-881 [2009]). Inset: sample traces from one neuron stimulated with 10 pulses at 5, 10, and 20 Hz (each trace is a 5-trial average). Colored underlines indicate the corresponding stim. train period. Dashed line indicates the time at which values ΔF/F values were measured (500 ms after stim. onset). b. Histogram of measured GCaMP3 fluorescence transient half-decay times following offset of a photostimulation epoch (τ1/2 calculated from single-exponential decay fits). Following stim. offset, transients evoked in cells returned to resting levels with off-kinetics (τ1/2 = 375+/-196 ms; mean +/- s.d.) in the range observed in vivo during trains of electrically stimulated APs (τ1/2 = 384+/-76 ms for 10 APs; Tian et al., Nat. Methods 6, 875-881 [2009]). c. Peak GCaMP3 transient amplitude during raster-scanning photostimulation of a cell using the spatial focusing path (SF in Fig. 1, main text) shown for different TPE raster-scan periods, which varied by changing the number of lines in a raster-scan, and which were repeated over an interval of 512 ms. The dashed line indicates the approximate C1V1(t/t) inactivation time-constant (τoff = 40-50 ms; Mattis et al., Nat. Methods 9, 159-172 [2012]; Prakash et al., Nat. Methods 9, 1171-1179 [2012]). Faster-scanning photostimulation trials (Ts< τoff) produced larger-amplitude responses than slower-scanning trials (Ts> τoff; n=31 target cells; values for each cell normalized by maximum amplitude in that cell). This relationship is a signature of membrane depolarization mediated by scanning recruitment of opsin probes (Rickgauer and Tank, PNAS 106, 15025-15030 [2009]; Prakash et al., Nat. Methods 9, 1171-1179 [2012]; Packer et al., Nat. Methods 9, 1202-1205 [2012]). Inset: Exemplary ΔF/F traces from one cell illustrating this relationship (colors indicate scan periods of same-color dots in panel; bars indicate s.d.).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 277 kb)
Supplementary Methods Checklist
(PDF 350 kb)
Rights and permissions
About this article
Cite this article
Rickgauer, J., Deisseroth, K. & Tank, D. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat Neurosci 17, 1816–1824 (2014). https://doi.org/10.1038/nn.3866
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3866
This article is cited by
-
High-speed multiplane confocal microscopy for voltage imaging in densely labeled neuronal populations
Nature Neuroscience (2023)
-
Cortico-cortical feedback engages active dendrites in visual cortex
Nature (2023)
-
Robust and adjustable dynamic scattering compensation for high-precision deep tissue optogenetics
Communications Biology (2023)
-
Neural signal propagation atlas of Caenorhabditis elegans
Nature (2023)
-
Prominent in vivo influence of single interneurons in the developing barrel cortex
Nature Neuroscience (2023)