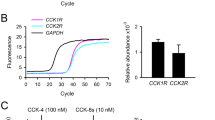
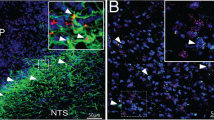
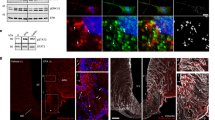
Abstract
Hypoglycemia initiates the counter-regulatory response (CRR), in which the sympathetic nervous system, glucagon and glucocorticoids restore glucose to appropriate concentrations. During starvation, low leptin levels restrain energy utilization, enhancing long-term survival. To ensure short-term survival during hypoglycemia in fasted animals, the CRR must overcome this energy-sparing program and nutrient depletion. Here we identify in mice a previously unrecognized role for leptin and a population of leptin-regulated neurons that modulate the CRR to meet these challenges. Hypoglycemia activates neurons of the parabrachial nucleus (PBN) that coexpress leptin receptor (LepRb) and cholecystokinin (CCK) (PBN LepRbCCK neurons), which project to the ventromedial hypothalamic nucleus. Leptin inhibits these cells, and Cckcre-mediated ablation of LepRb enhances the CRR. Inhibition of PBN LepRb cells blunts the CRR, whereas their activation mimics the CRR in a CCK-dependent manner. PBN LepRbCCK neurons are a crucial component of the CRR system and may be a therapeutic target in hypoglycemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
10 December 2014
In the version of this article initially published, the anteroposterior and dorsoventral stereotaxic injection coordinates in the Online Methods were transposed. The error has been corrected in the HTML and PDF versions of the article.
References
Levin, B.E., Dunn-Meynell, A.A. & Routh, V.H. CNS sensing and regulation of peripheral glucose levels. Int. Rev. Neurobiol. 51, 219–258 (2002).
McCrimmon, R.J. & Sherwin, R.S. Hypoglycemia in type 1 diabetes. Diabetes 59, 2333–2339 (2010).
Taborsky, G.J. Jr. Islets have a lot of nerve! Or do they? Cell Metab. 14, 5–6 (2011).
Sanders, N.M. et al. Feeding and neuroendocrine responses after recurrent insulin-induced hypoglycemia. Physiol. Behav. 87, 700–706 (2006).
Awoniyi, O., Rehman, R. & Dagogo-Jack, S. Hypoglycemia in patients with type 1 diabetes: epidemiology, pathogenesis, and prevention. Curr. Diab. Rep. 13, 669–678 (2013).
Ahima, R.S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 (1996).
Montague, C.T. et al. Congenital leptin deficiency is associated with severe early onset obesity in humans. Nature 387, 903–908 (1997).
Farooqi, I.S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Légrádi, G., Emerson, C.H., Ahima, R.S., Flier, J.S. & Lechan, R.M. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 138, 2569–2576 (1997).
Gautron, L. & Elmquist, J.K. Sixteen years and counting: an update on leptin in energy balance. J. Clin. Invest. 121, 2087–2093 (2011).
Ahima, R.S., Saper, C.B., Flier, J.S. & Elmquist, J.K. Leptin regulation of neuroendocrine systems. Front. Neuroendocrinol. 21, 263–307 (2000).
Morton, G.J., Cummings, D.E., Baskin, D.G., Barsh, G.S. & Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 443, 289–295 (2006).
Myers, M.G. Jr., Munzberg, H., Leinninger, G.M. & Leshan, R.L. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 9, 117–123 (2009).
Friedman, J.M. Leptin at 14 y of age: an ongoing story. Am. J. Clin. Nutr. 89, 973S–979S (2009).
McMinn, J.E. et al. Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am. J. Physiol. Endocrinol. Metab. 289, E403–E411 (2005).
de Luca, C. et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J. Clin. Invest. 115, 3484–3493 (2005).
Cohen, P. et al. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 108, 1113–1121 (2001).
Scott, M.M. et al. Leptin targets in the mouse brain. J. Comp. Neurol. 514, 518–532 (2009).
Patterson, C.M., Leshan, R.L., Jones, J.C. & Myers, M.G. Jr. Molecular mapping of mouse brain regions innervated by leptin receptor–expressing cells. Brain Res. 1378, 18–28 (2011).
Grill, H.J. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 14 (suppl. 5), 216S–221S (2006).
Ring, L.E. & Zeltser, L.M. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J. Clin. Invest. 120, 2931–2941 (2010).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Leshan, R.L., Greenwald-Yarnell, M., Patterson, C.M., Gonzalez, I.E. & Myers, M.G. Jr. Leptin action through hypothalamic nitric oxide synthase-1–expressing neurons controls energy balance. Nat. Med. 18, 820–823 (2012).
Hayes, M.R. et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 11, 77–83 (2010).
Scott, M.M., Williams, K.W., Rossi, J., Lee, C.E. & Elmquist, J.K. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Invest. 121, 2413–2421 (2011).
Huo, L., Gamber, K.M., Grill, H.J. & Bjorbaek, C. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology 149, 492–497 (2008).
Huo, L., Maeng, L., Bjorbaek, C. & Grill, H.J. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and Leptin. Endocrinology 148, 2189–2197 (2007).
Garfield, A.S. et al. Neurochemical characterization of body weight–regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology 153, 4600–4607 (2012).
Opland, D. et al. Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Mol Metab. 2, 423–434 (2013).
Levin, B.E., Magnan, C., Dunn-Meynell, A. & Le Foll, C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology 152, 2552–2557 (2011).
Levin, B.E., Becker, T.C., Eiki, J., Zhang, B.B. & Dunn-Meynell, A.A. Ventromedial hypothalamic glucokinase is an important mediator of the counterregulatory response to insulin-induced hypoglycemia. Diabetes 57, 1371–1379 (2008).
Chan, O. et al. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes 57, 1363–1370 (2008).
Barnes, M.B., Lawson, M.A. & Beverly, J.L. Rate of fall in blood glucose and recurrent hypoglycemia affect glucose dynamics and noradrenergic activation in the ventromedial hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1815–R1820 (2011).
Satoh, N. et al. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes 48, 1787–1793 (1999).
Takaki, A., Nagai, K., Takaki, S., Yanaihara, N. & Nakagawa, H. Satiety function of neurons containing a CCK-like substance in the dorsal parabrachial nucleus. Physiol. Behav. 48, 865–871 (1990).
Yoshimatsu, H., Egawa, M. & Bray, G.A. Effects of cholecystokinin on sympathetic activity to interscapular brown adipose tissue. Brain Res. 597, 298–303 (1992).
Le Lay, J. et al. CRTC2 (TORC2) contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose homeostasis. Cell Metab. 10, 55–62 (2009).
Krashes, M.J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Armbruster, B.N., Li, X., Pausch, M.H., Herlitze, S. & Roth, B.L. Evolving the lock to fit the key to create a family of G protein–coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 104, 5163–5168 (2007).
Wu, Q., Clark, M.S. & Palmiter, R.D. Deciphering a neuronal circuit that mediates appetite. Nature 483, 594–597 (2012).
Leinninger, G.M. et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 14, 313–323 (2011).
Song, P., Mabrouk, O.S., Hershey, N.D. & Kennedy, R.T. In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography–mass spectrometry. Anal. Chem. 84, 412–419 (2012).
Jiang, L., He, L. & Fountoulakis, M. Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J. Chromatogr. A 1023, 317–320 (2004).
Kawamori, D. et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 9, 350–361 (2009).
Jacobson, L., Ansari, T. & McGuinness, O.P. Counterregulatory deficits occur within 24 h of a single hypoglycemic episode in conscious, unrestrained, chronically cannulated mice. Am. J. Physiol. Endocrinol. Metab. 290, E678–E684 (2006).
Acknowledgements
We thank AstraZeneca Pharmaceuticals for the generous gift of leptin, B. Roth (University of North Carolina, Chapel Hill) for CNO and members of the labs of M.G.M. and L.K.H. for helpful discussions. Research support was provided by the Animal Phenotyping Core of the Michigan Diabetes Research Center (US National Institutes of Health (NIH) grant P30 DK020572) and the Michigan Nutrition and Obesity Research Center (P30 DK089503), the American Diabetes Association, the American Heart Association, the Marilyn H. Vincent Foundation and the NIH (DK098853) to M.G.M., NIH DK055267 and DK020595 to C.J.R., NIH DK046060 to R.T.K., NIH DK046409 to L.S. and the Wellcome Trust (098012) and Biotechnology and Biological Sciences Research Council (BB/K001418/1) to L.K.H.
Author information
Authors and Affiliations
Contributions
J.N.F. and C.M.P. produced the data in Figures 1,2,3,4,5 and Supplementary Figures 1,2,3,4,5,6,7,8,9,10, with the exception of the data in Figure 1e–i, which was produced by P.B.G. and L.S., and the measurements of catecholamines, which were performed by P.A.M., J.-M.T.W. and R.T.K. M.G. and M.R. helped produce Supplementary Figures 2, 5 and 10. J.C.J. aided with Figures 2,3,4 and with animal genotyping and husbandry. G.D., A.S.G. and L.K.H. performed the experiments in Figure 6. Adenoviral tracers were produced by A.K.S. and C.J.R. Experimental design, interpretation and manuscript preparation were led by M.G.M., L.K.H., J.N.F., C.M.P., A.S.G. and D.P.O.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Leptin-responsive LepRb neurons in PBN.
LepRbeYFP mice were injected with IP (a) vehicle or (b) leptin (5 mg/kg) 2 hours prior to perfusion and immunostaining for pSTAT3 (purple) and GFP (eYFP; green). Representative of 2 similar experiments, each with n = 3 animals. The PBN of representative mice is shown. Scale bars = 100 μM. Inset- digital zoom of the indicated area. Arrowheads indicate examples of colocalized neurons; not all colocalized neurons are indicated.
Supplementary Figure 2 Activation of PBN LepRb cells by IIH and glucoprivation but not i.c.v. insulin.
(a,b) LepRbeYFP mice were injected with IP (a) vehicle or 2DG (250 mg/kg) or (b) vehicle or insulin (1 U/kg) 2 hours prior to perfusion and immunostaining for cFos (purple) and GFP (eYFP; green) *p<0.001, {t(13) = −4.371}. (c,d) LepRbeYFP mice were injected with ICV (c) *p = 0.008, {t(12) = −3.19} vehicle or 2DG (1µg) *p<0.001, {t(9) = −6.01} or (d) vehicle or insulin (300mU) 2 hours prior to perfusion and immunostaining for cFos (purple) and GFP (eYFP; green). Colocalized cFos+eYFP and total eYFP neurons were quantified; data are presented as %colocalized/total eYFP. All studies: n = 5–8 animals analyzed by one tailed t-test; each panel representative of two separate studies.
Supplementary Figure 3 Similar pattern of cFos IR in PBN LepRb cells in response to i.p. insulin and 2DG.
LepRbeYFP mice were injected with IP (a) vehicle, (b) insulin (1 U/kg), or or 2DG (250 mg/kg) 2 hours prior to perfusion and immunostaining for cFos (purple) and GFP (eYFP; green). The PBN of representative mice is shown. Scale bars= 100 μM. n = 9 animals per group; representative of two separate studies. Inset- digital zoom of the indicated area. Arrowheads indicate examples of colocalized neurons; not all colocalized neurons are indicated.
Supplementary Figure 4 Leptin blunts fasting-induced enhancement of the CRR.
Male C57Bl6/J mice were allowed to feed ad libitum (Control), were fasted for 12 hours (Fast), or were injected with leptin (5 mg/kg, IP) and fasted for 12 hours (Fast+Leptin) before being injected with 2DG (500 mg/kg, IP). (a) Blood glucose was monitored at the indicated times; p = 0.013 (60 min), <0.001 (90 min), 0.008 (120 min) vs control and p = 0.003 (90 min), 0.008 (120 min) vs Fast + Leptin {F(12,26) = 4.61} by two way repeated measures ANOVA with Fisher LSD post hoc test. N = 8–9 animals per condition; data are plotted as mean +/− SEM. Letters indicate significant differences relative to other conditions by ANOVA with Fishers post-hoc test. (b) Area under the curve (AUC) for the data graphed in (a); *p<0.001 vs control and *0.009 vs Fast + Leptin {F(2,26) = 15.15}by one way ANOVA with Fisher LSD post hoc test.
Supplementary Figure 5 Induction of cFos in PBN CCK neurons by glucoprivation.
(a,b) CCKeYFP mice were injected with IP (a) vehicle or (b) 2DG (250 mg/kg, IP) 2 hours prior to perfusion and immunostaining for cFos (purple) and GFP (eYFP; green). The PBN of representative mice is shown. N=11 animals per condition; representative of two similar studies. Scale bars= 100 μM. Inset- digital zoom of the indicated area. Arrowheads indicate examples of colocalized neurons; not all colocalized neurons are indicated. (c) Quantification of the data. Colocalized cFos+eYFP and total eYFP neurons were quantified; data are presented as %colocalized/total eYFP; *p = 0.017, {t(16) = −2.32}. n = 7–11 animals per condition *p<0.05 by one tailed t-test; representative of two separate studies.
Supplementary Figure 6 Reduced leptin-stimulated pSTAT3 IR in PBN and EW nuclei of LepRbCck KO mice.
Quantitation of pSTAT3-IR cells in the indicated hindbrain nuclei of Control and LepRbCckKO mice following treatment with leptin (IP, 5 mg/kg) 2 hours prior to perfusion. N = 3–4 animals per condition; data are plotted as mean +/− SEM. NTS- nucleus tractus solitarius; PBN- parabrachial nucleus; PAG- periaqueductal grey; DR- dorsal raphe; EW- Edinger-Westphal; LR- linear raphe. EW: t(5) = 4.01, *p = 0.05. PBN: t(5) = 8.28, *p<0.001 by one tailed t-test.
Supplementary Figure 7 Energy balance and glucose homeostasis in LepRbCck KO mice.
(a) Weekly body weight for male Control and LepRbCckKO mice. (b) GTT (blood glucose values at the indicated times following an IP injection of 3 g/kg) in 15-week old male Control and LepRbCckKO mice. Hormone levels at 14 weeks of age in male Control and LepRbCckKO mice: (c) leptin, (d) insulin, (e) corticosterone. N = 12–18 animals per condition; p = NS for all comparisons; all data are plotted as mean +/− SEM.
Supplementary Figure 8 Fasting fails to enhance the CRR to glucoprivation in LepRbCck KO mice.
Male Control and LepRbCckKO mice were fasted for 4 (a,b) or 24 (c,d) hours prior to injection with 2DG (250 mg/kg, IP); blood glucose was monitored for 180 minutes afterwards (a,c); *p<0.001 (90 min), <0.001 (12 0min), {F(6,20) = 19.43} by two way repeated measures ANOVA with Fisher LSD post hoc test. (b,d) Quantification of area under the curve (AUC) from the data in (a,c) respectively; *p = 0.012, {t(19) = −2.79} by two tailed t-test. N = 9–13 animals per condition; all data are plotted as mean +/− SEM.
Supplementary Figure 9 Leptin action via PBNCck neurons does not alter glycemic responses to restraint stress.
(a) Leprcre mice were crossed onto the Rosa26-eYFP background to generate LepRbeYFP mice expressing eYFP in LepRb neurons. (b,c) LepRbeYFP mice were handled briefly (b) or subjected to restraint stress in a conical tube for 30 minutes (c) and then were perfused 2 hours prior to immunostaining for cFos (purple) and GFP (eYFP; green) in one experiment. The PBN of representative mice is shown. Inset- digital zoom of the indicated area. Arrowheads indicate examples of colocalized neurons. (d) Cckcre mice were crossed onto the Leprflox background to generate LepRbCckKO mice. (e,f) Control and LepRbCckKOeYFP mice were subjected to restraint stress for 30 minutes and blood glucose was monitored for 120 minutes (e). (f) Area under the curve (AUC) is plotted for the data in (e). N = 2–4 animals per condition; p = NS for all comparisons; all data are plotted as mean +/− SEM.
Supplementary Figure 10 Induction of cFos in PBN and VMH neurons after DREADD-mediated activation of PBN LepRb neurons.
The cre-inducible AAV-hM3Dq was injected bilaterally into the PBN of male Leprcre mice. Animals were injected with Vehicle (a,c) or CNO (b,d; 0.3 mg/kg, IP) and monitored for 120 minutes (see Figure 6). At the end of the experiment, animals were perfused and brain sections were stained for cFos (green; panels a-d) and/or dsRed (red; panels a-b). Representative images are shown for the PBN (a,b) and VMH (c,d) in one experiment. (e) Quantification of cFos in the VMH of Vehicle and CNO treated animals. N = 9–11 animals per condition; *p<0.001, {t(18) = −6.83} by one-tailed t-test; all data are plotted as mean +/− SEM.
Supplementary Figure 11 Working model of PBN LepRb neurons.
Our data suggest that PBN LepRb expressing neurons are excited by hypoglycemia, inhibited by leptin, release CCK in the VMH to drive counterregulatory responses. Our data indicates the importance of this pathway in modulating counter-regulatory responses during fasting conditions to promote survival of the individual.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 1397 kb)
Supplementary Methods Checklist
(PDF 421 kb)
Rights and permissions
About this article
Cite this article
Flak, J., Patterson, C., Garfield, A. et al. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci 17, 1744–1750 (2014). https://doi.org/10.1038/nn.3861
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3861
This article is cited by
-
A parabrachial-hypothalamic parallel circuit governs cold defense in mice
Nature Communications (2023)
-
Arcuate AgRP, but not POMC neurons, modulate paraventricular CRF synthesis and release in response to fasting
Cell & Bioscience (2022)
-
The ventromedial hypothalamic nucleus: watchdog of whole-body glucose homeostasis
Cell & Bioscience (2022)
-
Lipid biosynthesis enzyme Agpat5 in AgRP-neurons is required for insulin-induced hypoglycemia sensing and glucagon secretion
Nature Communications (2022)
-
Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding
Nature Metabolism (2022)