Abstract
The temperature of an object provides important somatosensory information for animals performing tactile tasks. Humans can perceive skin cooling of less than one degree, but the sensory afferents and central circuits that they engage to enable the perception of surface temperature are poorly understood. To address these questions, we examined the perception of glabrous skin cooling in mice. We found that mice were also capable of perceiving small amplitude skin cooling and that primary somatosensory (S1) cortical neurons were required for cooling perception. Moreover, the absence of the menthol-gated transient receptor potential melastatin 8 ion channel in sensory afferent fibers eliminated the ability to perceive cold and the corresponding activation of S1 neurons. Our results identify parts of a neural circuit underlying cold perception in mice and provide a new model system for the analysis of thermal processing and perception and multimodal integration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Frenzel, H. et al. A genetic basis for mechanosensory traits in humans. PLoS Biol. 10, e1001318 (2012).
Johnson, K.O., Darian-Smith, I. & LaMotte, C. Peripheral neural determinants of temperature discrimination in man: a correlative study of responses to cooling skin. J. Neurophysiol. 36, 347–370 (1973).
McKemy, D.D. The molecular and cellular basis of cold sensation. ACS Chem. Neurosci. 4, 238–247 (2013).
Bautista, D.M. et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208 (2007).
Dhaka, A. et al. TRPM8 is required for cold sensation in mice. Neuron 54, 371–378 (2007).
Colburn, R.W. et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron 54, 379–386 (2007).
Feldmeyer, D. et al. Barrel cortex function. Prog. Neurobiol. 103, 3–27 (2013).
Diamond, M.E. et al. 'Where' and 'what' in the whisker sensorimotor system. Nat. Rev. Neurosci. 9, 601–612 (2008).
Sachidhanandam, S., Sreenivasan, V., Kyriakatos, A., Kremer, Y. & Petersen, C.C.H. Membrane potential correlates of sensory perception in mouse barrel cortex. Nat. Neurosci. 16, 1671–1677 (2013).
Guo, Z.V. et al. Flow of cortical activity underlying a tactile decision in mice. Neuron 81, 179–194 (2014).
Miyashita, T. & Feldman, D.E. Behavioral detection of passive whisker stimuli requires somatosensory cortex. Cereb. Cortex 23, 1655–1662 (2013).
Hutson, K.A. & Masterton, R.B. The sensory contribution of a single vibrissa's cortical barrel. J. Neurophysiol. 56, 1196–1223 (1986).
Kleinfeld, D. & Deschênes, M. Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron 72, 455–468 (2011).
Finger, S. & Frommer, G.P. Effects of cortical and thalamic lesions on temperature discrimination and responsiveness to foot shock in the rat. Brain Res. 24, 69–89 (1970).
Finger, S., Scheff, S., Warshaw, I. & Cohen, K. Retention and acquisition of fine temperature discriminations following somatosensory cortical lesions in the rat. Exp. Brain Res. 10, 340–346 (1970).
Downer, J. & Zubek, J.P. Role of the cerebral cortex in temperature discrimination in the rat. J. Comp. Physiol. Psychol. 47, 199–203 (1954).
Porter, L.H., Hecht, G.S. & Sheaffer, R. Disturbances in the performance of thermal discrimination tasks following cortical ablations in rats. Brain Res. 621, 319–330 (1993).
Hellon, R.F., Misra, N.K. & Provins, K.A. Neurones in the somatosensory cortex of the rat responding to scrotal skin temperature changes. J. Physiol. (Lond.) 232, 401–411 (1973).
Duclaux, R. & Kenshalo, D.R. The temperature sensitivity of the type I slowly adapting mechanoreceptors in cats and monkeys. J. Physiol. (Lond.) 224, 647–664 (1972).
Schepers, R.J. & Ringkamp, M. Thermoreceptors and thermosensitive afferents. Neurosci. Biobehav. Rev. 33, 205–212 (2009).
Campero, M., Serra, J., Bostock, H. & Ochoa, J.L. Slowly conducting afferents activated by innocuous low temperature in human skin. J. Physiol. (Lond.) 535, 855–865 (2001).
Zimmermann, K. et al. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 108, 18114–18119 (2011).
Noël, J. et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 28, 1308–1318 (2009).
Barth, A.L. & Poulet, J.F.A. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 35, 345–355 (2012).
Komiyama, T. et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464, 1182–1186 (2010).
McKemy, D.D., Neuhausser, W.M. & Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 (2002).
Peier, A.M. et al. A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 (2002).
Knibestöl, M. & Vallbo, A.B. Single-unit analysis of mechanoreceptor activity from the human glabrous skin. Acta Physiol. Scand. 80, 178–195 (1970).
Lewin, G.R. & Moshourab, R. Mechanosensation and pain. J. Neurobiol. 61, 30–44 (2004).
Marshall, J. Sensory disturbances in cortical wounds with special reference to pain. J. Neurol. Neurosurg. Psychiatry 14, 187–204 (1951).
Veldhuijzen, D.S., Greenspan, J.D., Kim, J.H. & Lenz, F.A. Altered pain and thermal sensation in subjects with isolated parietal and insular cortical lesions. Eur. J. Pain 14, 535 e1–11 (2010).
Adams, R.W. & Burke, D. Deficits of thermal sensation in patients with unilateral cerebral lesions. Electroencephalogr. Clin. Neurophysiol. 73, 443–452 (1989).
Cushing, H. A note upon the faradic stimulation of the postcentral gyrus in conscious patients. Brain 32, 44–53 (1909).
Penfield, W. & Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443 (1937).
Duclaux, R., Franzen, O., Chatt, A.B., Kenshalo, D.R. & Stowell, H. Responses recorded from human scalp evoked by cutaneous thermal stimulation. Brain Res. 78, 279–290 (1974).
Landgren, S. Cortical reception of cold impulses from the tongue of the cat. Acta Physiol. Scand. 40, 202–209 (1957).
Tsuboi, Y. et al. Response properties of primary somatosensory cortical neurons responsive to cold stimulation of the facial skin and oral mucous membrane. Brain Res. 613, 193–202 (1993).
Kreisman, N.R. & Zimmerman, I.D. Representation of information about skin temperature in the discharge of single cortical neurons. Brain Res. 55, 343–353 (1973).
Birklein, F., Rolke, R. & Müller-Forell, W. Isolated insular infarction eliminates contralateral cold, cold pain, and pinprick perception. Neurology 65, 1381 (2005).
Cattaneo, L., Chierici, E., Cucurachi, L., Cobelli, R. & Pavesi, G. Posterior insular stroke causing selective loss of contralateral nonpainful thermal sensation. Neurology 68, 237 (2007).
Mazzola, L., Isnard, J., Peyron, R. & Mauguière, F. Stimulation of the human cortex and the experience of pain: Wilder Penfield's observations revisited. Brain 135, 631–640 (2012).
Maihöfner, C., Kaltenhäuser, M., Neundörfer, B. & Lang, E. Temporo-spatial analysis of cortical activation by phasic innocuous and noxious cold stimuli—a magnetoencephalographic study. Pain 100, 281–290 (2002).
Craig, A.D., Chen, K., Bandy, D. & Reiman, E.M. Thermosensory activation of insular cortex. Nat. Neurosci. 3, 184–190 (2000).
Rodgers, K.M., Benison, A.M., Klein, A. & Barth, D.S. Auditory, somatosensory and multisensory insular cortex in the rat. Cereb. Cortex 18, 2941–2951 (2008).
Mackenzie, R.A., Burke, D., Skuse, N.F. & Lethlean, A.K. Fibre function and perception during cutaneous nerve block. J. Neurol. Neurosurg. Psychiatry 38, 865–873 (1975).
Green, B.G. Localization of thermal sensation: an illusion and synthetic heat. Percept. Psychophys. 22, 331–337 (1977).
Stevens, J.C. & Green, B.G. Temperature-touch interaction: Weber's phenomenon revisited. Sens. Processes 2, 206–209 (1978).
Iurilli, G. et al. Sound-driven synaptic inhibition in primary visual cortex. Neuron 73, 814–828 (2012).
Senkowski, D., Schneider, T.R., Foxe, J.J. & Engel, A.K. Crossmodal binding through neural coherence: implications for multisensory processing. Trends Neurosci. 31, 401–409 (2008).
Saleem, A.B., Ayaz, A., Jeffery, K.J., Harris, K.D. & Carandini, M. Integration of visual motion and locomotion in mouse visual cortex. Nat. Neurosci. 16, 1864–1869 (2013).
Poulet, J.F.A. & Petersen, C.C.H. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–885 (2008).
Stürzebecher, A.S. et al. An in vivo tethered toxin approach for the cell-autonomous inactivation of voltage-gated sodium channel currents in nociceptors. J. Physiol. (Lond.) 588, 1695–1707 (2010).
Milenkovic, N. et al. Nociceptive tuning by stem cell factor/c-Kit signaling. Neuron 56, 893–906 (2007).
Koltzenburg, M., Stucky, C.L. & Lewin, G.R. Receptive properties of mouse sensory neurons innervating hairy skin. J. Neurophysiol. 78, 1841–1850 (1997).
Acknowledgements
We thank J. König for technical assistance, J.-S. Jouhanneau for help with cell reconstruction, L. Estebanez for programming advice, and L. Estebanez and E. Bobrov for constructive comments on a previous version of the manuscript. This work was funded by grants from the European Research Council (ERC-2010-StG-260590, J.F.A.P.; ERC-2011-StG-280565, J.S.) and the Deutsche Forschungs Gemeinschaft (Exc 257 NeuroCure and DFG-FOR-1341 BaCoFun, J.F.A.P.; Collaborative Research Center 665 Project B6, G.R.L.). Additional support was obtained from a Bernstein Center for Computational Neuroscience grant from the BMBF (01GQ1001E, G.R.L.).
Author information
Authors and Affiliations
Contributions
N.M., J.F.A.P. and G.R.L. designed the project. N.M. performed and analyzed behavioral training, intrinsic optical imaging, whole-cell recordings and pharmacological inactivation. W.-J.Z. performed whole-cell recordings. J.W. performed and analyzed afferent recordings. T.A. wrote code and performed behavioral training. J.S. provided reagents and the Trpm8 mice. J.F.A.P. and G.R.L. supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
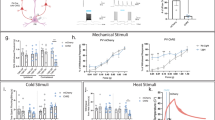
Supplementary Figure 1 Examples of learning curves and lick timing from individual mice during thermal detection task.
a, Examples of individual learning curves from 3 mice, bold cyan line showing the correct hit rate, light cyan the false licks. Mice were trained one session/day. b, Peri-stimulus time histograms (PSTHs) of licking during stimulus presentation from day 5 from the corresponding mice in a. Thermal stimulus onset is at time = 0s and lasted for 3 s. Top PSTHs shows the successful hits, bottom PSTHs in light cyan show the false licks.
Supplementary Figure 2 A cold responsive L2/3 excitatory cortical pyramidal neuron in mouse forepaw S1.
a, Anatomical reconstruction of a cortical pyramidal neuron in mouse forepaw somatosensory cortex. b, Single trial examples of the response of the same cell to cold stimulation of the paw under isoflurane anesthesia with averaged membrane potential response underneath and PSTH on bottom to 40 presentations of a cooling stimulus. Horizontal marks on Vm represent –60 mV for single trials and –74 mV for the averaged response.
Supplementary Figure 3 Mouse forepaw does not move during cooling thermal stimulation under isoflurane anesthesia.
a, b, c, Three example, averaged Layer 2/3 cortical whole-cell recordings (black traces) from different mice during cold-thermal (32-22°C) stimulation of the forepaw (blue trace). the distance of paw movement (orange) was monitored with a movement sensor arm resting on top of the forepaw digits while the force was kept constant (green). The movement sensor was sensitive enough to record a slow (about 3 s duration) movement of the peltier element during cooling that moved the paw by about 1 μm. This slight movement did not cause responses in cortex as (i) cortical control recordings showed no response, data not shown; (ii) the same stimulator was used for single nerve afferent fibers recordings and did not evoked tactile responses in low threshold mechanoreceptors; (iii) the cold response starts before movement onset in the middle cell, see vertical red dashed line in (b). Horizontal marks next to Vm represent, (a) –56 mV, (b) –64 mV, (c) –64 mV.
Supplementary Figure 4 Layer 2/3 cortical neurons in Trpm8–/– mice respond to tactile stimulation of the forepaw.
a, b, c, Three example cells from different mice showing significant averaged subthreshold responses (magenta) to 100 Hz vibrotactile stimulation (orange) of the forepaw digits.
Supplementary Figure 5 Trpm8–/– mice are able to learn an acoustic detection task.
a, cartoon schematic of head-restrained mouse undergoing acoustic training with water rewards. b, 2 Trpm8–/– mice were trained to lick in response to brief (5 ms) acoustic stimulus presentation at randomized times directly after cooling detection training. These Trpm8–/– mice were not able to learn to report mild cooling, they learned to lick after the acoustic stimulus already in the first trial. On the forth session, the mean hit rate was 86.1 ± 3.0% (mean in purple) compared to false licks 8.5 ± 3.5% (mean in black). Error bars show s.e.m.
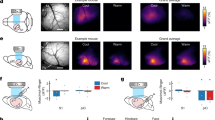
Supplementary Figure 6 Primary sensory afferent recordings from Trpm8+/+ and Trpm8–/– mice during a 10 °C cooling stimulus
a, Example recordings from cutaneous C-fibers in WT (blue) and Trpm8–/– (magenta) mice during cold stimulation matching the stimulus used during behavioral training (32-22°C). Inset shows colored spikes that were selected for analysis, and in grey the discarded spikes. b, Temperature threshold for the first spike in all fibers responding to cold. In good agreement with the dataset in Figure 6, very few cold responsive A-β or A-δ fibers were identified with the 10°C cooling stimulus. In comparison, a large number of C-MHC (C-MechanoHeatCold) and C-MC (C-MechanoCold) fibers with low response threshold were identified. The C-fiber population showed a large reduction in Trpm8–/– mice. Grey dot shows a unimodal, cold specific C-fiber with similar threshold to other cold sensitive C-fibers. Horizontal bars represent mean with s.e.m..
Supplementary Figure 7 Population analysis of all fibers recorded in entire dataset from Trpm8–/– and Trpm8+/+ mice
a, From the entire surveyed population, 15.6% (24/154 single units) responded to cooling in Trpm8+/+ mice but only 5.7% (11/193 single units) in the Trpm8–/– mice (Chi-squared test P = 0.0024). There was no change in the proportion of heating sensitive afferents in the Trpm8–/– mouse. b From the four subtypes of C recorded (C-M, C-Mechano; C-MH, C-MechanoHeat; C-MC, C-MechanoCold, C-MHC, C-MechanoHeatCold), there was a significant reduction in the numbers of recorded C-MC (Chi-squared test P = 0.0025) and no C-MHCs were identified, but there was no change in the numbers of in C-M and C-MH in the Trpm8–/– mouse. c, We recorded 5 major types of A fiber (A-β; A-δ; A-βC; A-δC; A-δH) none of which showed significant changes in numbers in the Trpm8–/– mouse.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 468 kb)
Rights and permissions
About this article
Cite this article
Milenkovic, N., Zhao, WJ., Walcher, J. et al. A somatosensory circuit for cooling perception in mice. Nat Neurosci 17, 1560–1566 (2014). https://doi.org/10.1038/nn.3828
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3828
This article is cited by
-
The secondary somatosensory cortex gates mechanical and heat sensitivity
Nature Communications (2024)
-
The brain’s encoding of warm and cool
Nature (2023)
-
The cellular coding of temperature in the mammalian cortex
Nature (2023)
-
USH2A is a Meissner’s corpuscle protein necessary for normal vibration sensing in mice and humans
Nature Neuroscience (2021)
-
Automated and rapid self-report of nociception in transgenic mice
Scientific Reports (2020)