Abstract
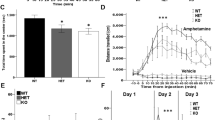
Children with neurofibromatosis type 1 (NF1) are increasingly recognized as having a high prevalence of social difficulties and autism spectrum disorders (ASDs). We demonstrated a selective social learning deficit in mice with deletion of a single Nf1 allele (Nf1+/−), along with greater activation of the mitogen-activated protein kinase pathway in neurons from the amygdala and frontal cortex, structures that are relevant to social behaviors. The Nf1+/− mice showed aberrant amygdala glutamate and GABA neurotransmission, deficits in long-term potentiation and specific disruptions in the expression of two proteins that are associated with glutamate and GABA neurotransmission: a disintegrin and metalloprotease domain 22 (Adam22) and heat shock protein 70 (Hsp70), respectively. All of these amygdala disruptions were normalized by the additional deletion of the p21 protein-activated kinase (Pak1) gene. We also rescued the social behavior deficits in Nf1+/− mice with pharmacological blockade of Pak1 directly in the amygdala. These findings provide insights and therapeutic targets for patients with NF1 and ASDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Johnson, N.S., Saal, H.M., Lovell, A.M. & Schorry, E.K. Social and emotional problems in children with neurofibromatosis type 1: evidence and proposed interventions. J. Pediatr. 134, 767–772 (1999).
Barton, B. & North, K. Social skills of children with neurofibromatosis type 1. Dev. Med. Child Neurol. 46, 553–563 (2004).
Noll, R.B. et al. Social, emotional, and behavioral functioning of children with NF1. Am. J. Med. Genet. A. 143A, 2261–2273 (2007).
Lehtonen, A., Howie, E., Trump, D. & Huson, S.M. Behaviour in children with neurofibromatosis type 1: cognition, executive function, attention, emotion, and social competence. Dev. Med. Child Neurol. 55, 111–125 (2013).
Huijbregts, S.C. & de Sonneville, L.M. Does cognitive impairment explain behavioral and social problems of children with neurofibromatosis type 1? Behav. Genet. 41, 430–436 (2011).
Huijbregts, S., Jahja, R., De Sonneville, L., de Breij, S. & Swaab-Barneveld, H. Social information processing in children and adolescents with neurofibromatosis type 1. Dev. Med. Child Neurol. 52, 620–625 (2010).
Garg, S. et al. Neurofibromatosis type 1 and autism spectrum disorder. Pediatrics 132, e1642–e1648 (2013).
Garg, S. et al. Autism and other psychiatric comorbidity in neurofibromatosis type 1: evidence from a population-based study. Dev. Med. Child Neurol. 55, 139–145 (2013).
Walsh, K.S. et al. Symptomatology of autism spectrum disorder in a population with neurofibromatosis type 1. Dev. Med. Child Neurol. 55, 131–138 (2013).
Pride, N.A. et al. The genetic and neuroanatomical basis of social dysfunction: lessons from neurofibromatosis type 1. Hum. Brain Mapp. 35, 2372–2382 (2014).
Truitt, W.A. et al. From anxiety to autism: spectrum of abnormal social behaviors modeled by progressive disruption of inhibitory neuronal function in the basolateral amygdala in Wistar rats. Psychopharmacology (Berl.) 191, 107–118 (2007).
Maaswinkel, H., Baars, A.M., Gispen, W.H. & Spruijt, B.M. Roles of the basolateral amygdala and hippocampus in social recognition in rats. Physiol. Behav. 60, 55–63 (1996).
Todd, R.M. & Anderson, A.K. Six degrees of separation: the amygdala regulates social behavior and perception. Nat. Neurosci. 12, 1217–1218 (2009).
Zhu, Y., Ghosh, P., Charnay, P., Burns, D.K. & Parada, L.F. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science 296, 920–922 (2002).
Costa, R.M. et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415, 526–530 (2002).
Cui, Y. et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell 135, 549–560 (2008).
Sankoorikal, G.M., Kaercher, K.A., Boon, C.J., Lee, J.K. & Brodkin, E.S. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol. Psychiatry 59, 415–423 (2006).
Crawley, J.N. et al. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides 41, 145–163 (2007).
Brittain, J.M. et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca2+ channel complex. Nat. Med. 17, 822–829 (2011).
Le, L.Q. & Parada, L.F. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene 26, 4609–4616 (2007).
Wang, Y. et al. ERK inhibition rescues defects in fate specification of Nf1-deficient neural progenitors and brain abnormalities. Cell 150, 816–830 (2012).
Zhang, Y.Y. et al. Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J. Exp. Med. 187, 1893–1902 (1998).
Li, C., Dabrowska, J., Hazra, R. & Rainnie, D.G. Synergistic activation of dopamine D1 and TrkB receptors mediate gain control of synaptic plasticity in the basolateral amygdala. PLoS ONE 6, e26065 (2011).
Deacon, S.W. et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 15, 322–331 (2008).
Kalwat, M.A., Yoder, S.M., Wang, Z. & Thurmond, D.C. A p21-activated kinase (PAK1) signaling cascade coordinately regulates F-actin remodeling and insulin granule exocytosis in pancreatic β cells. Biochem. Pharmacol. 85, 808–816 (2013).
Fukata, Y. et al. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science 313, 1792–1795 (2006).
Ohkawa, T. et al. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J. Neurosci. 33, 18161–18174 (2013).
Fukata, Y. et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc. Natl. Acad. Sci. USA 107, 3799–3804 (2010).
Hsu, C.C. et al. Association of l-glutamic acid decarboxylase to the 70-kDa heat shock protein as a potential anchoring mechanism to synaptic vesicles. J. Biol. Chem. 275, 20822–20828 (2000).
Sagane, K. et al. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 6, 33 (2005).
Hubberstey, A.V. & Mottillo, E.P. Cyclase-associated proteins: CAPacity for linking signal transduction and actin polymerization. FASEB J. 16, 487–499 (2002).
Yang, F.C. et al. Nf1-dependent tumors require a microenvironment containing Nf1+/−– and c-kit–dependent bone marrow. Cell 135, 437–448 (2008).
Nakatani, N. et al. Expression analysis of actin-related genes as an underlying mechanism for mood disorders. Biochem. Biophys. Res. Commun. 352, 780–786 (2007).
Dolan, B.M. et al. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc. Natl. Acad. Sci. USA 110, 5671–5676 (2013).
Martin, I. et al. Transmission disequilibrium study of an oligodendrocyte and myelin glycoprotein gene allele in 431 families with an autistic proband. Neurosci. Res. 59, 426–430 (2007).
Marui, T. et al. Association between the neurofibromatosis-1 (NF1) locus and autism in the Japanese population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 131B, 43–47 (2004).
Sturm, V. et al. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front. Hum. Neurosci. 6, 341 (2012).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates (Academic Press, 2008).
Porsolt, R.D., Le Pichon, M. & Jalfre, M. Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732 (1977).
Johnson, P.L., Truitt, W.A., Fitz, S.D., Lowry, C.A. & Shekhar, A. Neural pathways underlying lactate-induced panic. Neuropsychopharmacology 33, 2093–2107 (2008).
Mann, B. et al. ProteinQuant Suite: a bundle of automated software tools for label-free quantitative proteomics. Rapid Commun. Mass Spectrom. 22, 3823–3834 (2008).
Molosh, A.I. et al. NPY Y1 receptors differentially modulate GABAA and NMDA receptors via divergent signal transduction pathways to reduce excitability of amygdala neurons. Neuropsychopharmacology 38, 1352–1364 (2013).
McDonald, A.J., Mascagni, F., Mania, I. & Rainnie, D.G. Evidence for a perisomatic innervation of parvalbumin-containing interneurons by individual pyramidal cells in the basolateral amygdala. Brain Res. 1035, 32–40 (2005).
Acknowledgements
This work was supported by grants from the National Center for Advanced Translational Sciences, US National Institutes of Health UL1RR025761/TR000006, R01 MH52619 and MH065702 (to A.S.), a predoctoral fellowship to J.P.S. (TL1 RR 025759), K01AG044466 (to P.L.J.) and R01 CA74177-06 (to D.W.C.). We thank T. Jacks (Massachusetts Institute of Technology) for providing Nf1+/− mice and J. Chernoff (Fox Chase Cancer Center) for providing Pak1−/− mice.
Author information
Authors and Affiliations
Contributions
A.S., along with A.I.M., P.L.J., J.P.S. and D.W.C., formulated the hypotheses and designed the studies. S.-J.P. and D.W.C. maintained the mouse colony and genotyped all mice. J.P.S. and S.P.J. performed the behavioral assays shown in Figure 1, and J.P.S. performed those shown in Figure 2. For Figure 1 and Supplementary Figure 3, western blots were performed by R.K. and W.Z., and immunohistochemistry was performed by L.M.F. and P.L.J. A.I.M. performed all electrophysiology experiments shown in Figure 3. For Table 1 and Figure 4a,b and Supplementary Table 1, A.I.M. micropunched the BLA, Y.S.M. and Z.M.S. conducted the proteomics assay, and C.G., L.L. and P.L.J. analyzed the data. The immunohistochemistry shown in Figure 4c–f was performed by L.M.F. and P.L.J. The stereotaxic surgeries and behavioral assays shown in Figure 5 were performed by D.A. and C.B. The stereotaxic surgeries and immunohistochemistry shown in Supplementary Figure 2 were done by D.A. and P.L.J. A.I.M. and P.L.J. analyzed data and created figures. A.I.M., P.L.J., J.P.S., D.W.C. and A.S. interpreted the data and collectively wrote the main draft of the article, with all other authors contributing to the revisions, and all authors approved of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
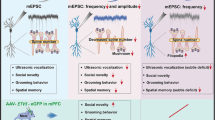
Supplementary Figure 1 Hypothetical graphical illustrations of Nf1 and Pak1 gene pathways and glutamate and GABA regulation of electrophysiological activity in the basolateral amygdala of WT and Nf1+/– mice.
(a) The hypothetical graphical illustration, pathways adapted from Le and Parada (2007)1, showing the interaction of Nf1 and Pak1 gene products in growth factor signal transduction. Neurofibromin, a cytoplasmic GAP-like protein, negatively regulates RAS activation by accelerating the conversion of RAS-GTP to RAS-GDP and increasing RAS–RAF-MEK signal transduction, whereas Pak1 has an activating effect on this pathway. (b) is a hypothetical illustration depicting glutamate and GABA regulation of normal EPSCs, LTP and IPSCs activity in WT mice (top illustration), and how in Nf1+/- mice (bottom illustration) disruption of ADAM22 interrupts anchoring of AMPA receptors to the post-synaptic membrane and leads to non-sustainable LTP as demonstrated in Figure 3h. Similarly, increased expression of HSP70 could contribute to increases in pre-synaptic GABA release and increased frequency of IPSCs seen in Figure 3c. Abbreviations: BLA, basolateral amygdala; CeA, central amygdala
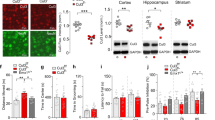
Supplementary Figure 2 Effects of a Pak1 inhibitor on DMSO-induced increases in pErk expression within the basolateral amygdala
(a)Bilateral injections of 100% DMSO + the inactive enantiomer PIR3.5 (a pharmacological control for IPA3, 100 μM/100 nl) into the BLA of WT mice induced a robust increase in the no. of local pERK-ir cells, which was attenuated in WT mice that were injected with 100% DMSO + the PAK1 inhibitor IPA3 (100 μM/100 nl: F2,11 = 22.6, p < 0.001, n = 5,3,4). (b) Bilateral injections of 100% DMSO + the inactive enantiomer PIR3.5 (a pharmacological control for IPA3, 100 μM/100 nl) into the BLA of WT mice also reduced in the no. of local ADAM22-ir cells, which was attenuated in WT mice that were injected with 100% DMSO + the PAK1 inhibitor IPA3 (100 μM/100 nl: F2,11 = 22.6, p < 0.001, n = 5,3,4). (c) is a plotted linear regression that illustrates that the no. of ADAM22-ir cells in the BLA is inversely correlated with the no. of pERK-ir cells in the BLA in WT mice from experiment in SFig- 1a-b. (d) contains photomicrographs of pERK and ADAM22-immunoreactive cells in the amygdala from each treatment group. Scale bar is 25 μm. (e) is an illustration of a coronal mouse brain section (taken from Franklin & Paxinos stereotaxic atlas of the mouse brain, 3rd edition) indicating the location of the injection sites for each treatment group within the BLA from -1.46 to -1.94 mm bregma with a 0.5mm scale bar at bottom right. Abbreviations: CeA, central amygdala; ec, external capsule; LA, lateral amygdala; opt, optic tract.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Table 1 (PDF 1410 kb)
Rights and permissions
About this article
Cite this article
Molosh, A., Johnson, P., Spence, J. et al. Social learning and amygdala disruptions in Nf1 mice are rescued by blocking p21-activated kinase. Nat Neurosci 17, 1583–1590 (2014). https://doi.org/10.1038/nn.3822
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3822
This article is cited by
-
The therapeutic potential of neurofibromin signaling pathways and binding partners
Communications Biology (2023)
-
Social circuits and their dysfunction in autism spectrum disorder
Molecular Psychiatry (2023)
-
Differential epitranscriptome and proteome modulation in the brain of neonatal mice exposed to isoflurane or sevoflurane
Cell Biology and Toxicology (2023)
-
Non-invasive brain stimulation modulates GABAergic activity in neurofibromatosis 1
Scientific Reports (2022)
-
The p21-activated kinases in neural cytoskeletal remodeling and related neurological disorders
Protein & Cell (2022)