Abstract
A fundamental problem in neuroscience is how emotional valences are represented in the brain. We know little about how appetitive and aversive systems interact and the extent to which information regarding these two opposite values segregate and converge. Here we used a new method, tyramide-amplified immunohistochemistry–fluorescence in situ hybridization, to simultaneously visualize the neural correlates of two stimuli of contrasting emotional valence across the limbic forebrain at single-cell resolution. We discovered characteristic patterns of interaction, segregated, convergent and intermingled, between the appetitive and aversive neural ensembles in mice. In nucleus accumbens, we identified a mosaic activation pattern by positive and negative emotional cues, and unraveled previously unappreciated functional heterogeneity in the D1- and D2-type medium-spiny neurons, which correspond to the Go and NoGo pathways. These results provide insights into the coding of emotional valence in the brain and act as a proof of principle of a powerful methodology for simultaneous functional mapping of two distinct behaviors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
LeDoux, J.E. The Emotional Brain: the Mysterious Underpinnings of Emotional Life (Simon & Schuster, New York, 1996).
Panksepp, J. Affective neuroscience: the foundations of human and animal emotions. (Oxford University Press, New York, 1998).
Lang, P.J. & Davis, M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog. Brain Res. 156, 3–29 (2006).
Chen, X., Gabitto, M., Peng, Y., Ryba, N.J. & Zuker, C.S. A gustotopic map of taste qualities in the mammalian brain. Science 333, 1262–1266 (2011).
Marshall, W.H., Woolsey, C.N. & Bard, P. Observations on cortical somatic sensory mechanisms of cat and monkey. J. Neurophysiol. 4, 1–24 (1941).
Mombaerts, P. et al. Visualizing an olfactory sensory map. Cell 87, 675–686 (1996).
LeDoux, J. Rethinking the emotional brain. Neuron 73, 653–676 (2012).
Anderson, D.J. & Adolphs, R. A framework for studying emotions across species. Cell 157, 187–200 (2014).
Johnson, Z.V., Revis, A.A., Burdick, M.A. & Rhodes, J.S. A similar pattern of neuronal Fos activation in 10 brain regions following exposure to reward- or aversion-associated contextual cues in mice. Physiol. Behav. 99, 412–418 (2010).
Freed, P.J., Yanagihara, T.K., Hirsch, J. & Mann, J.J. Neural mechanisms of grief regulation. Biol. Psychiatry 66, 33–40 (2009).
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012).
Matsumoto, M. & Hikosaka, O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841 (2009).
Morrison, S.E. & Salzman, C.D. The convergence of information about rewarding and aversive stimuli in single neurons. J. Neurosci. 29, 11471–11483 (2009).
Roitman, M.F., Wheeler, R.A. & Carelli, R.M. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 45, 587–597 (2005).
Shabel, S.J. & Janak, P.H. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc. Natl. Acad. Sci. USA 106, 15031–15036 (2009).
Taha, S.A. & Fields, H.L. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J. Neurosci. 25, 1193–1202 (2005).
Chaudhuri, A., Nissanov, J., Larocque, S. & Rioux, L. Dual activity maps in primate visual cortex produced by different temporal patterns of zif268 mRNA and protein expression. Proc. Natl. Acad. Sci. USA 94, 2671–2675 (1997).
Chan, R.K.W., Brown, E.R., Ericsson, A., Kovacs, K.J. & Sawchenko, P.E. A comparison of 2 immediate-early genes, c-Fos and Ngfi-B, as markers for functional activation in stress-related neuroendocrine circuitry. J. Neurosci. 13, 5126–5138 (1993).
Greenberg, M.E. & Ziff, E.B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature 311, 433–438 (1984).
Morgan, J.I., Cohen, D.R., Hempstead, J.L. & Curran, T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science 237, 192–197 (1987).
Farivar, R., Zangenehpour, S. & Chaudhuri, A. Cellular-resolution activity mapping of the brain using immediate-early gene expression. Front. Biosci. 9, 104–109 (2004).
Gerfen, C.R. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu. Rev. Neurosci. 15, 285–320 (1992).
Kreitzer, A.C. Physiology and pharmacology of striatal neurons. Annu. Rev. Neurosci. 32, 127–147 (2009).
Surmeier, D.J., Ding, J., Day, M., Wang, Z. & Shen, W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 30, 228–235 (2007).
Bechara, A. & van der Kooy, D. Opposite motivational effects of endogenous opioids in brain and periphery. Nature 314, 533–534 (1985).
Ciocchi, S. et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010).
Haubensak, W. et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276 (2010).
Koob, G.F. & Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 (2010).
Sawchenko, P.E., Li, H.Y. & Ericsson, A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog. Brain Res. 122, 61–78 (2000).
Sheehan, T.P., Chambers, R.A. & Russell, D.S. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res. Brain Res. Rev. 46, 71–117 (2004).
Bertran-Gonzalez, J. et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor–expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 28, 5671–5685 (2008).
Guzowski, J.F., McNaughton, B.L., Barnes, C.A. & Worley, P.F. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 2, 1120–1124 (1999).
Dölen, G., Darvishzadeh, A., Huang, K.W. & Malenka, R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013).
Kreitzer, A.C. & Malenka, R.C. Striatal plasticity and basal ganglia circuit function. Neuron 60, 543–554 (2008).
Russo, S.J. et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 33, 267–276 (2010).
Salamone, J.D. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav. Brain Res. 61, 117–133 (1994).
Sesack, S.R. & Grace, A.A. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47 (2010).
Wheeler, R.A. & Carelli, R.M. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology 56, 149–159 (2009).
Reynolds, S.M. & Berridge, K.C. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J. Neurosci. 22, 7308–7320 (2002).
Hikida, T., Kimura, K., Wada, N., Funabiki, K. & Nakanishi, S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66, 896–907 (2010).
Kravitz, A.V., Tye, L.D. & Kreitzer, A.C. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci. 15, 816–818 (2012).
Lee, K.W. et al. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor–containing medium spiny neurons in nucleus accumbens. Proc. Natl. Acad. Sci. USA 103, 3399–3404 (2006).
Kuffler, S.W. Discharge patterns and functional organization of mammalian retina. J. Neurophysiol. 16, 37–68 (1953).
Taverna, S., Ilijic, E. & Surmeier, D.J. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. J. Neurosci. 28, 5504–5512 (2008).
Schultz, W., Dayan, P. & Montague, P.R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Scicli, A.P., Petrovich, G.D., Swanson, L.W. & Thompson, R.F. Contextual fear conditioning is associated with lateralized expression of the immediate early gene c-fos in the central and basolateral amygdalar nuclei. Behav. Neurosci. 118, 5–14 (2004).
Switzman, L., Hunt, T. & Amit, Z. Heroin and morphine: aversive and analgesic effects in rats. Pharmacol. Biochem. Behav. 15, 755–759 (1981).
Lin, D. et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011).
Reijmers, L.G., Perkins, B.L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007).
Guenthner, C.J., Miyamichi, K., Yang, H.H., Heller, H.C. & Luo, L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784 (2013).
Acknowledgements
We thank J. Feldman for critical review of the manuscript, S. Sarah, H. Kessels, A. Roe and M. Poo for comments on the manuscript, and S. Song, J. Huang, B. Lu and members of the Hu laboratory for stimulating discussions. This work was supported by the Chinese 973 Program (2011CBA00400), the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB02030004), the One Hundred Talents Program and the Outstanding Youth Grant (to H.H.).
Author information
Authors and Affiliations
Contributions
J.X. designed the study and performed the TAI-FISH experiments. Q.Z. and Tao Zhou contributed to the TAI-FISH experiments. Ting-ting Zhou tested the time course for chocolate stimulation. Y.C. performed the statistical analysis. H.H. conceived the idea, designed the study and wrote the manuscript with input from J.X., Q.Z. and Tao Zhou.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Time course of c-fos mRNA and protein expression in CEA (a), PVN (b) and NAc (c) (related to Figs. 2–4)
The schematic was adapted from the mouse brain atlas (Paxinos and Franklin, 2001). Arrows indicate the time points chosen for the dual labeling experiment in Figures 2,3,4. TAI-FISH was used for the 4h and 6h time points in (c) as indicated by the circles. I-FISH was used for all other time points. n = 3 mice/group. Error bars represent s.e.m.
Supplementary Figure 2 Time course of c-fos mRNA and protein expression in basolateral amygdala (BLA) (a), medial prefrontal cortex (mPFC) (b) and dorsal raphe (DR) (c)
Note that no optimal time points were available for dual labeling analysis in these brain regions, due to persistent mRNA signals. All time points were examined by I-FISH. TAI-FISH was also examined for the 6h time points, but the mRNA signals were still present (data not shown). Error bars represent s.e.m.
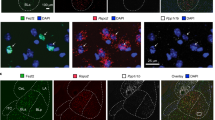
Supplementary Figure 3 Representative images showing the double labeling (I-FISH for CEA and PVN, TAI-FISH for NAc) for c-fos protein (green channel) and mRNA (red channel) following single stimulation in CEA (a), PVN (b) and NAc (c) (related to Figs. 2–4)
0 min indicates the time point of animal sacrifice. Scale bars are 100 μm in a, 50 μm in b and c.
Supplementary Figure 4 MEA is activated by foot shock, but not morphine
a. Schematic illustrating the structure of MEA and its neighboring regions. Red box indicates the position of the image taken in (b). b. Representative images showing I-FISH double labeling for c-fos protein (green) and mRNA (red) in MEA following single stimulation of Mor or FS, and sequential stimulation of Mor-FS. Scale bars = 50 μm.
Supplementary Figure 5 BSTov is activated by morphine, but not foot shock
a.Schematic illustrating the structure of BSTov and its neighboring regions. Red box indicates the position of the image taken in (b). VP, ventral pallidum. b. Representative images showing the green (c-fos protein) and red (c-fos mRNA) channels of TAI-FISH double labeling in BSTov following single stimulation of Mor or FS, and sequential stimulation of Mor-FS. Scale bars = 50 μm.
Supplementary Figure 6 Partially convergent neural representations of morphine and foot shock in BSTfu
a. Schematic illustrating the structure of BSTfu and its neighboring regions. Red box indicates the position of the image taken in (b) and (c). b. Representative images showing the green (c-fos protein) and red (c-fos mRNA) channels of TAI-FISH double labeling in BSTfu following single stimulation of Mor or FS. c. Representative images showing the green (c-fos protein), red (c-fos mRNA) and merged channels of double labeling from five experiments: Sal-Ctx, Mor-Ctx, Sal-FS, Mor-Mor, and Mor-FS. Scale bars = 50 μm. d-e. Percentage of neurons expressing c-fos protein (d) and mRNA (e) in BSTfu in the six experimental conditions: Sal-Ctx, Mor-Ctx, Sal-FS, Mor-Mor, Mor-FS, and Mor-Coc. f. Percentage of overlap in the Mor-FS, Mor-Mor and Mor-Coc double labeling experiments. n = 3 mice/group. More than 250 neurons per mouse from 3 mice were counted for each group.Paired t-test adjusted by Benjamini-Hochberg procedure controlling the false discovery rate. *, p<0.05; **, p<0.01. Error bars represent s.e.m.
Supplementary Figure 7 Intermingled neural representations of morphine and foot shock in LSv
a. Schematic illustrating the structure of LSv and its neighboring regions. Red box indicates the position of the image taken in B and C. b. Representative images showing the green (c-fos protein) and red (c-fos mRNA) channels of TAI-FISH double labeling in LSv following single stimulation of Mor or FS. c. Representative images showing the green (c-fos protein), red (c-fos mRNA) and merged channels of double labeling from five experiments: Sal-Ctx, Mor-Ctx, Sal-FS, Mor-Mor, and Mor-FS. Scale bars = 50 μm. d-e Percentage of neurons expressing c-fos protein (d) and mRNA (e) in LSv in the six experimental conditions: Sal-Ctx, Mor-Ctx, Sal-FS, Mor-Mor, Mor-FS, and Mor-Coc. Note that the second morphine- and cocaine-induced mRNA signals were significantly reduced, presumably due to strong desensitization in this region. f. Percentage of overlap in the Mor-FS, Mor-Mor and Mor-Coc double labeling experiments. n = 3 mice/group. More than 250 neurons per mouse from 3 mice were counted for each group. Paired t-test adjusted by Benjamini-Hochberg procedure controlling the false discovery rate. *,p<0.05. Error bars represent s.e.m.
Supplementary Figure 8 Summary of neural representations for morphine and foot shock in different regions of limbic forebrain, as revealed by I-FISH and TAI-FISH in this study
Scale bars are 200 μm.
Supplementary Figure 9 Morphine did not alter the averseness of foot shock
a. Example trajectory plot of two representative mice, which received either saline or morphine 6 hours earlier, and were tested by the real-time place preference assay for 20 min. They received foot-shock every time they entered the shock chamber on the left. b. Quantification of the number of attempts mice tried to enter the shock chamber during the 20-min period, n = 4 mice each group, Mann-Whitney U test, n.s., not significant, P = 0.69. Error bars represent s.e.m.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 3949 kb)
Rights and permissions
About this article
Cite this article
Xiu, J., Zhang, Q., Zhou, T. et al. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat Neurosci 17, 1552–1559 (2014). https://doi.org/10.1038/nn.3813
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3813
This article is cited by
-
Hippocampal cells segregate positive and negative engrams
Communications Biology (2022)
-
A distinct D1-MSN subpopulation down-regulates dopamine to promote negative emotional state
Cell Research (2022)
-
Top-down regulation of motivated behaviors via lateral septum sub-circuits
Molecular Psychiatry (2022)
-
Medial preoptic area antagonistically mediates stress-induced anxiety and parental behavior
Nature Neuroscience (2021)
-
Projections from D2 Neurons in Different Subregions of Nucleus Accumbens Shell to Ventral Pallidum Play Distinct Roles in Reward and Aversion
Neuroscience Bulletin (2021)