Abstract
Hyperalgesia arising from sensitization of pain relays in the spinal dorsal horn shares many mechanistic and phenotypic parallels with memory formation. We discovered that mechanical hyperalgesia could be rendered labile and reversible in mice after reactivation of spinal pain pathways in a process analogous to memory reconsolidation. These findings reveal a previously unknown regulatory mechanism underlying hyperalgesia and demonstrate the existence of reconsolidation-like processes in a sensory system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Latremoliere, A. & Woolf, C.J. J. Pain 10, 895–926 (2009).
Sandkühler, J. Physiol. Rev. 89, 707–758 (2009).
Ji, R.R., Kohno, T., Moore, K.A. & Woolf, C.J. Trends Neurosci. 26, 696–705 (2003).
Sandkühler, J. & Lee, J. Trends Neurosci. 36, 343–352 (2013).
Nader, K., Schafe, G.E. & Le Doux, J.E. Nature 406, 722–726 (2000).
Debiec, J., LeDoux, J.E. & Nader, K. Neuron 36, 527–538 (2002).
Ruscheweyh, R., Wilder-Smith, O., Drdla, R., Liu, X.-G. & Sandkühler, J. Mol. Pain 7, 20 (2011).
Agarwal, N., Offermanns, S. & Kuner, R. Genesis 38, 122–129 (2004).
Daou, I. et al. J. Neurosci. 33, 18631–18640 (2013).
O'Neill, J. et al. Pharmacol. Rev. 64, 939–971 (2012).
Drdla, R., Gassner, M., Gingl, E. & Sandkuhler, J. Science 325, 207–210 (2009).
Ferrini, F. et al. Nat. Neurosci. 16, 183–192 (2013).
Tronson, N.C. & Taylor, J.R. Nat. Rev. Neurosci. 8, 262–275 (2007).
Fonseca, R., Nägerl, U.V. & Bonhoeffer, T. Nat. Neurosci. 9, 478–480 (2006).
Liu, X. & Sandkuhler, J. J. Neurophysiol. 78, 1973–1982 (1997).
Schouenborg, J. J. Physiol. (Lond.) 356, 169–192 (1984).
Ikeda, H. et al. Science 312, 1659–1662 (2006).
Drdla-Schutting, R., Benrath, J., Wunderbaldinger, G. & Sandkühler, J. Science 335, 235–238 (2012).
Bonin, R.P., Bories, C. & De Koninck, Y. Mol. Pain 10, 26 (2014).
Shields, S.D. et al. Pain 153, 2017–2030 (2012).
Chiu, I.M. et al. Nature 501, 52–57 (2013).
Todd, A.J. Nat. Rev. Neurosci. 11, 823–836 (2010).
Acknowledgements
We thank M. Desrochers-Couture and L.J. Martin for their assistance and advice with the behavioral assays. This work was supported by a Pfizer–Fonds de recherche Québec–Santé (FRQS) Innovation Fund Award to Y.D.K., an FRQS post-doctoral Fellowship to R.P.B., Canadian Institutes of Health Research grant MOP 12942 to Y.D.K., and the Catherine Bushnell Pain Research Fellowship from the Louise and Alan Edwards foundation to R.P.B.
Author information
Authors and Affiliations
Contributions
R.P.B. conducted all of the experiments and analyses. R.P.B. and Y.D.K. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
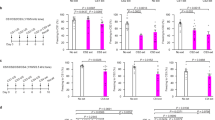
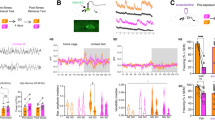
Supplementary Figure 1 Labile plasticity in spinal pain pathways enables reversal of hyperalgesia
(a) Intrathecal injection of anisomycin (Aniso) immediately prior to a single intraplantar injection of capsaicin (Cap) prevents the development of mechanical hyperalgesia 3 h after injection (Post-Cap). n = 6 mice per group. (b) Changes in mechanical withdrawal thresholds induced by intraplantar injection of capsaicin (t = 0 h) followed by a second ipsilateral intraplantar injection of Cap (t = 3 h). The intrathecal (i.t.) injection of Aniso or Veh at 2h15 ± 5 min after the second Cap injection did not alter hyperalgesia. (c) Changes in mechanical withdrawal thresholds induced by intraplantar injection of capsaicin (t = 0 h) followed by a second ipsilateral intraplantar injection of Cap or Veh and intraperitoneal (i.p.) injection of Aniso or Veh (t = 3 h). n = 6 mice per group except Veh + Aniso n = 5. (d) Summary of antihyperalgesia induced by the treatments in (c), expressed as percentage of maximum possible effect (MPE). (e) Changes in mechanical withdrawal thresholds induced by intraplantar injection of Cap (t = 0 h) followed by a second ipsilateral intraplantar injection of Cap or Veh and intrathecal (i.t.) injection of cycloheximide (CHX; t = 3 h). MPE: CHX + Veh = 25.7% ± 9.0%; CHX + Cap = 80.2% ± 22.0%; P = 0.045; n = 6 mice per group. (f) Low frequency (2 Hz) optical stimulation of a hind paw for 20 min in anesthetized Nav1.8+-ChR2 mice induces a transient mechanical hyperalgesia. Data shown as difference in withdraw threshold between stimulated (stim) and unstimulated (control) paw. *** indicates P < 0.001 at 1 h. n = 12 mice per group. (g) Plot of minimum intensity of light required to induce paw withdrawal from 488 nm light by Nav1.8+-ChR2 mice receiving intraplantar injection of Cap (t = 0 h) followed by light-induced sensitization (2 Hz, 20 min; Light) or sham stimulation (Sham) and intrathecal injection of Aniso or Veh (t = 3 h). n = 6 mice per group. (h–j) Capsaicin-induced hyperalgesia followed by intrathecal injection of: (h) AMPA ± Aniso, (i) NMDA ± Aniso, (j) Sar9,Met(O2)11-Substance P (SP) ± Aniso. (k) Summary of results from (h–j) expressed as MPE. n = 6 mice per group, except SP + Aniso: n = 5 mice, AMPA + Aniso: n = 12 mice. (l) Long term potentiation of post-synaptic field potentials (fPSPs) in the superficial dorsal horn induced by 2 Hz electrical stimulation (black arrow). n = 12 and 10 experiments from 6 and 5 mice in Control and APV, respectively. *, **, *** indicates P < 0.05, P < 0.01, and P < 0.001, respectively. All data are mean ± s.e.m.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Table 1 (PDF 571 kb)
Rights and permissions
About this article
Cite this article
Bonin, R., De Koninck, Y. A spinal analog of memory reconsolidation enables reversal of hyperalgesia. Nat Neurosci 17, 1043–1045 (2014). https://doi.org/10.1038/nn.3758
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3758
This article is cited by
-
Dynamic Changes of the Infralimbic Cortex and Its Regulation of the Prelimbic Cortex in Rats with Chronic Inflammatory Pain
Neuroscience Bulletin (2024)
-
Central and peripheral contributions of T-type calcium channels in pain
Molecular Brain (2022)
-
Nociception induces a differential presynaptic modulation of the synaptic efficacy of nociceptive and proprioceptive joint afferents
Experimental Brain Research (2021)
-
Differential chloride homeostasis in the spinal dorsal horn locally shapes synaptic metaplasticity and modality-specific sensitization
Nature Communications (2020)
-
Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents
Nature Medicine (2017)