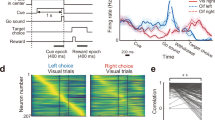
Abstract
The function of a given brain region is often defined by the coding properties of its individual neurons, yet how this information is combined at the ensemble level is an equally important consideration. We recorded multiple neurons from the anterior cingulate cortex (ACC) and the dorsal striatum (DS) simultaneously as rats performed different sequences of the same three actions. Sequence and lever decoding was markedly similar on a per-neuron basis in the two regions. At the ensemble level, sequence-specific representations in the DS appeared synchronously, but transiently, along with the representation of lever location, whereas these two streams of information appeared independently and asynchronously in the ACC. As a result, the ACC achieved superior ensemble decoding accuracy overall. Thus, the manner in which information was combined across neurons in an ensemble determined the functional separation of the ACC and DS on this task.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Duncan, J. An adaptive coding model of neural function in prefrontal cortex. Nat. Rev. Neurosci. 2, 820–829 (2001).
Jung, M.W., Qin, Y., McNaughton, B.L. & Barnes, C.A. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb. Cortex 8, 437–450 (1998).
Fino, E. & Yuste, R. Dense inhibitory connectivity in neocortex. Neuron 69, 1188–1203 (2011).
Packer, A.M. et al. Two-photon optogenetics of dendritic spines and neural circuits. Nat. Methods 9, 1202–1205 (2012).
Durstewitz, D., Vittoz, N.M., Floresco, S.B. & Seamans, J.K. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron 66, 438–448 (2010).
Rich, E.L. & Shapiro, M. Rat prefrontal cortical neurons selectively code strategy switches. J. Neurosci. 29, 7208–7219 (2009).
Parthasarathy, H.B. & Graybiel, A.M. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J. Neurosci. 17, 2477–2491 (1997).
Koós, T. & Tepper, J.M. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci. 2, 467–472 (1999).
Gage, G.J., Stoetzner, C.R., Wiltschko, A.B. & Berke, J.D. Selective activation of striatal fast-spiking interneurons during choice execution. Neuron 67, 466–479 (2010).
Berke, J.D., Okatan, M., Skurski, J. & Eichenbaum, H.B. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43, 883–896 (2004).
Graybiel, A.M., Aosaki, T., Flaherty, A.W. & Kimura, M. The basal ganglia and adaptive motor control. Science 265, 1826–1831 (1994).
Graybiel, A.M. Building action repertoires: memory and learning functions of the basal ganglia. Curr. Opin. Neurobiol. 5, 733–741 (1995).
Averbeck, B.B. & Lee, D. Effects of noise correlations on information encoding and decoding. J. Neurophysiol. 95, 3633–3644 (2006).
Sesack, S.R., Deutch, A.Y., Roth, R.H. & Bunney, B.S. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 290, 213–242 (1989).
Zheng, T. & Wilson, C.J. Corticostriatal combinatorics: the implications of corticostriatal axonal arborizations. J. Neurophysiol. 87, 1007–1017 (2002).
Mushiake, H., Saito, N., Sakamoto, K., Itoyama, Y. & Tanji, J. Activity in the lateral prefrontal cortex reflects multiple steps of future events in action plans. Neuron 50, 631–641 (2006).
Averbeck, B.B. & Lee, D. Prefrontal neural correlates of memory for sequences. J. Neurosci. 27, 2204–2211 (2007).
Shima, K., Isoda, M., Mushiake, H. & Tanji, J. Categorization of behavioural sequences in the prefrontal cortex. Nature 445, 315–318 (2007).
Procyk, E., Tanaka, Y.L. & Joseph, J.P. Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat. Neurosci. 3, 502–508 (2000).
Barone, P. & Joseph, J.P. Prefrontal cortex and spatial sequencing in macaque monkey. Exp. Brain Res. 78, 447–464 (1989).
Nakamura, K., Sakai, K. & Hikosaka, O. Neuronal activity in medial frontal cortex during learning of sequential procedures. J. Neurophysiol. 80, 2671–2687 (1998).
Ninokura, Y., Mushiake, H. & Tanji, J. Integration of temporal order and object information in the monkey lateral prefrontal cortex. J. Neurophysiol. 91, 555–560 (2004).
Shidara, M. & Richmond, B.J. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science 296, 1709–1711 (2002).
Ryou, J.W. & Wilson, F.A. Making your next move: dorsolateral prefrontal cortex and planning a sequence of actions in freely moving monkeys. Cogn. Affect. Behav. Neurosci. 4, 430–443 (2004).
Lu, X. & Ashe, J. Anticipatory activity in primary motor cortex codes memorized movement sequences. Neuron 45, 967–973 (2005).
Schmitzer-Torbert, N. & Redish, A.D. Neuronal activity in the rodent dorsal striatum in sequential navigation: separation of spatial and reward responses on the multiple T task. J. Neurophysiol. 91, 2259–2272 (2004).
Fujii, N. & Graybiel, A.M. Time-varying covariance of neural activities recorded in striatum and frontal cortex as monkeys perform sequential-saccade tasks. Proc. Natl. Acad. Sci. USA 102, 9032–9037 (2005).
Fujii, N. & Graybiel, A.M. Representation of action sequence boundaries by macaque prefrontal cortical neurons. Science 301, 1246–1249 (2003).
Aldridge, J.W. & Berridge, K.C. Coding of serial order by neostriatal neurons: a “natural action” approach to movement sequence. J. Neurosci. 18, 2777–2787 (1998).
Seo, M., Lee, E. & Averbeck, B.B. Action selection and action value in frontal-striatal circuits. Neuron 74, 947–960 (2012).
Cowen, S.L. & McNaughton, B.L. Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. J. Neurophysiol. 98, 303–316 (2007).
Euston, D.R. & McNaughton, B.L. Apparent encoding of sequential context in rat medial prefrontal cortex is accounted for by behavioral variability. J. Neurosci. 26, 13143–13155 (2006).
Krzanowski, W.J. Principles of Multivariate Analysis: a User's Perspective (Oxford University Press, 2000).
Berdyyeva, T.K. & Olson, C.R. Rank signals in four areas of macaque frontal cortex during selection of actions and objects in serial order. J. Neurophysiol. 104, 141–159 (2010).
Clower, W.T. & Alexander, G.E. Movement sequence-related activity reflecting numerical order of components in supplementary and presupplementary motor areas. J. Neurophysiol. 80, 1562–1566 (1998).
Grillner, S., Hellgren, J., Menard, A., Saitoh, K. & Wikstrom, M.A. Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci. 28, 364–370 (2005).
Carrillo-Reid, L. et al. Encoding network states by striatal cell assemblies. J. Neurophysiol. 99, 1435–1450 (2008).
Calabresi, P., Misgeld, U. & Dodt, H.U. Intrinsic membrane properties of neostriatal neurons can account for their low level of spontaneous activity. Neuroscience 20, 293–303 (1987).
Wilson, C.J. & Kawaguchi, Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 16, 2397–2410 (1996).
Hyman, J.M., Ma, L., Balaguer-Ballester, E., Durstewitz, D. & Seamans, J.K. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc. Natl. Acad. Sci. USA 109, 5086–5091 (2012).
Acknowledgements
This research was supported by Canadian Institutes of Health Research grants (MOP-93784 and MOP-84319).
Author information
Authors and Affiliations
Contributions
J.K.S. and L.M. designed the study, L.M. conducted the experiments, L.M., J.M.H., A.J.L. and J.K.S. performed data analysis and created the figures, A.G.P. helped interpret the results and write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
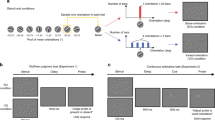
Supplementary Figure 1 Task description and performance.
a) The operant chamber contained 3 levers installed on the front panel and a food-cup on the opposing wall. A unique sensory cue was attached to the floor immediately in front of each lever as well as on the surrounding wall. The 1st lever (light gray in schematic) to be pressed in a given sequence block could have Velcro attached, the 2nd lever (dark gray in schematic) cardboard attached and the 3rd lever (black in schematic), soft foam attached. b) Example of a typical test day where the rat had to perform a minimum of 10 trials on each of the 3 sequence blocks, which were given in a pseudorandom order. On this session, sequence block A required the rat to respond on the right lever, followed by the middle lever and then the left lever, before reward pellets were delivered to the food-cup on the opposite wall. The serial order of the 3 sensory cues (velcro, cardboard, foam) remained constant for a given rat but were moved to different levers for each of the sequence blocks.
Supplementary Figure 2 Histology showing recording sites.
a) Histology showing representative electrode track endings (white arrows) in the ACC. b) Schematics showing the location and range of the recording sites in the ACC. Given that not all electrodes left a track, the recording range was inferred based on the visible tracks and the size of the electrode arrays. c) Representative electrode track endings (white arrows) in the DS. d) Schematics showing the location and range of the recording sites in the DS.
Supplementary Figure 3 Firing properties in the ACC and DS.
The iFRs from all bins and neurons were combined for each region, and their probability density functions were plotted on a logarithmic scale. Although both ACC and DS neurons usually have low firing rates, DS neurons exhibited brief periods of high activity more often than did ACC neurons, giving rise to the long-tailed firing rate distribution (DS: gray, ACC: black).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 393 kb)
Rights and permissions
About this article
Cite this article
Ma, L., Hyman, J., Lindsay, A. et al. Differences in the emergent coding properties of cortical and striatal ensembles. Nat Neurosci 17, 1100–1106 (2014). https://doi.org/10.1038/nn.3753
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3753
This article is cited by
-
Characteristics of the Neuronal Support for Operative Behavior Formed by Mono- and Multistep Methods
Neuroscience and Behavioral Physiology (2020)
-
Conflict and adaptation signals in the anterior cingulate cortex and ventral tegmental area
Scientific Reports (2018)
-
Fluid network dynamics in the prefrontal cortex during multiple strategy switching
Nature Communications (2018)