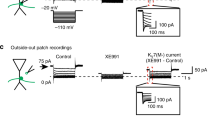
Abstract
Fast-spiking, parvalbumin-expressing GABAergic interneurons, a large proportion of which are basket cells (BCs), have a key role in feedforward and feedback inhibition, gamma oscillations and complex information processing. For these functions, fast propagation of action potentials (APs) from the soma to the presynaptic terminals is important. However, the functional properties of interneuron axons remain elusive. We examined interneuron axons by confocally targeted subcellular patch-clamp recording in rat hippocampal slices. APs were initiated in the proximal axon ∼20 μm from the soma and propagated to the distal axon with high reliability and speed. Subcellular mapping revealed a stepwise increase of Na+ conductance density from the soma to the proximal axon, followed by a further gradual increase in the distal axon. Active cable modeling and experiments with partial channel block revealed that low axonal Na+ conductance density was sufficient for reliability, but high Na+ density was necessary for both speed of propagation and fast-spiking AP phenotype. Our results suggest that a supercritical density of Na+ channels compensates for the morphological properties of interneuron axons (small segmental diameter, extensive branching and high bouton density), ensuring fast AP propagation and high-frequency repetitive firing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Freund, T.F. & Katona, I. Perisomatic inhibition. Neuron 56, 33–42 (2007).
Buzsáki, G. & Eidelberg, E. Commissural projection to the dentate gyrus of the rat: evidence for feed-forward inhibition. Brain Res. 230, 346–350 (1981).
Pouille, F. & Scanziani, M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163 (2001).
McBain, C.J. & Fisahn, A. Interneurons unbound. Nat. Rev. Neurosci. 2, 11–23 (2001).
Bartos, M., Vida, I. & Jonas, P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 8, 45–56 (2007).
Cardin, J.A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
de Almeida, L., Idiart, M. & Lisman, J.E. A second function of gamma frequency oscillations: an E%-max winner-take-all mechanism selects which cells fire. J. Neurosci. 29, 7497–7503 (2009).
Miles, R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J. Physiol. (Lond.) 428, 61–77 (1990).
Nörenberg, A., Hu, H., Vida, I., Bartos, M. & Jonas, P. Distinct nonuniform cable properties optimize rapid and efficient activation of fast-spiking GABAergic interneurons. Proc. Natl. Acad. Sci. USA 107, 894–899 (2010).
Hille, B. Ion Channels of Excitable Membrane (Sinauer, Sunderland, Massachusetts, 2001).
Debanne, D., Campanac, E., Bialowas, A., Carlier, E. & Alcaraz, G. Axon physiology. Physiol. Rev. 91, 555–602 (2011).
Hu, H., Martina, M. & Jonas, P. Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science 327, 52–58 (2010).
Shu, Y., Hasenstaub, A., Duque, A., Yu, Y. & McCormick, D.A. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature 441, 761–765 (2006).
Halasy, K. & Somogyi, P. Subdivisions in the multiple GABAergic innervation of granule cells in the dentate gyrus of the rat hippocampus. Eur. J. Neurosci. 5, 411–429 (1993).
Colbert, C.M. & Johnston, D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J. Neurosci. 16, 6676–6686 (1996).
Palmer, L.M. & Stuart, G.J. Site of action potential initiation in layer 5 pyramidal neurons. J. Neurosci. 26, 1854–1863 (2006).
Kim, S., Guzman, S.J., Hu, H. & Jonas, P. Active dendrites support efficient initiation of dendritic spikes in hippocampal CA3 pyramidal neurons. Nat. Neurosci. 15, 600–606 (2012).
Baranauskas, G., David, Y. & Fleidervish, I.A. Spatial mismatch between the Na+ flux and spike initiation in axon initial segment. Proc. Natl. Acad. Sci. USA 110, 4051–4056 (2013).
Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8, 451–465 (2007).
Meeks, J.P. & Mennerick, S. Action potential initiation and propagation in CA3 pyramidal axons. J. Neurophysiol. 97, 3460–3472 (2007).
Schmidt-Hieber, C., Jonas, P. & Bischofberger, J. Action potential initiation and propagation in hippocampal mossy fibre axons. J. Physiol. (Lond.) 586, 1849–1857 (2008).
Kole, M.H.P. et al. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 11, 178–186 (2008).
Mainen, Z.F., Joerges, J., Huguenard, J.R. & Sejnowski, T.J. A model of spike initiation in neocortical pyramidal neurons. Neuron 15, 1427–1439 (1995).
Hodgkin, A.L. & Huxley, A.F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. (Lond.) 116, 449–472 (1952).
Goldman, L. & Schauf, C.L. Quantitative description of sodium and potassium currents and computed action potentials in Myxicola giant axons. J. Gen. Physiol. 61, 361–384 (1973).
Sigworth, F.J. The variance of sodium current fluctuations at the node of Ranvier. J. Physiol. (Lond.) 307, 97–129 (1980).
Lorincz, A. & Nusser, Z. Cell type–dependent molecular composition of the axon initial segment. J. Neurosci. 28, 14329–14340 (2008).
Hu, W. et al. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nat. Neurosci. 12, 996–1002 (2009).
Fleidervish, I.A., Lasser-Ross, N., Gutnick, M.J. & Ross, W.N. Na+ imaging reveals little difference in action potential–evoked Na+ influx between axon and soma. Nat. Neurosci. 13, 852–860 (2010).
Schmidt-Hieber, C. & Bischofberger, J. Fast sodium channel gating supports localized and efficient axonal action potential initiation. J. Neurosci. 30, 10233–10242 (2010).
Boiko, T. et al. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J. Neurosci. 23, 2306–2313 (2003).
Carter, B.C. & Bean, B.P. Sodium entry during action potentials of mammalian neurons: incomplete inactivation and reduced metabolic efficiency in fast-spiking neurons. Neuron 64, 898–909 (2009).
Alle, H., Roth, A. & Geiger, J.R.P. Energy-efficient action potentials in hippocampal mossy fibers. Science 325, 1405–1408 (2009).
Hodgkin, A.L. & Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. (Lond.) 117, 500–544 (1952).
Wang, X.J. & Buzsáki, G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J. Neurosci. 16, 6402–6413 (1996).
Rudy, B. & McBain, C.J. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 24, 517–526 (2001).
Madeja, M. Do neurons have a reserve of sodium channels for the generation of action potentials? A study on acutely isolated CA1 neurons from the guinea-pig hippocampus. Eur. J. Neurosci. 12, 1–7 (2000).
Debanne, D., Guérineau, N.C., Gähwiler, B.H. & Thompson, S.M. Action-potential propagation gated by an axonal IA-like K+ conductance in hippocampus. Nature 389, 286–289 (1997).
Soleng, A.F., Chiu, K. & Raastad, M. Unmyelinated axons in the rat hippocampus hyperpolarize and activate an H current when spike frequency exceeds 1 Hz. J. Physiol. (Lond.) 552, 459–470 (2003).
Meeks, J.P. & Mennerick, S. Selective effects of potassium elevations on glutamate signaling and action potential conduction in hippocampus. J. Neurosci. 24, 197–206 (2004).
Moore, J.W., Stockbridge, N. & Westerfield, M. On the site of impulse initiation in a neurone. J. Physiol. (Lond.) 336, 301–311 (1983).
Colbert, C.M. & Pan, E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat. Neurosci. 5, 533–538 (2002).
Huxley, A.F. & Stämpfli, R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J. Physiol. (Lond.) 108, 315–339 (1949).
Engel, D. & Jonas, P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron 45, 405–417 (2005).
Lapray, D. et al. Behavior-dependent specialization of identified hippocampal interneurons. Nat. Neurosci. 15, 1265–1271 (2012).
Martina, M., Schultz, J.H., Ehmke, H., Monyer, H. & Jonas, P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J. Neurosci. 18, 8111–8125 (1998).
Okaty, B.W., Miller, M.N., Sugino, K., Hempel, C.M. & Nelson, S.B. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J. Neurosci. 29, 7040–7052 (2009).
Bartos, M. et al. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc. Natl. Acad. Sci. USA 99, 13222–13227 (2002).
Maex, R. & De Schutter, E. Resonant synchronization in heterogeneous networks of inhibitory neurons. J. Neurosci. 23, 10503–10514 (2003).
Traub, R.D., Whittington, M.A., Stanford, I.M. & Jefferys, J.G.R. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 383, 621–624 (1996).
Bischofberger, J., Engel, D., Li, L., Geiger, J.R.P. & Jonas, P. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat. Protoc. 1, 2075–2081 (2006).
Nevian, T., Larkum, M.E., Polsky, A. & Schiller, J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat. Neurosci. 10, 206–214 (2007).
Kraushaar, U. & Jonas, P. Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse. J. Neurosci. 20, 5594–5607 (2000).
Eggermann, E. & Jonas, P. How the “slow” Ca2+ buffer parvalbumin affects transmitter release in nanodomain coupling regimes at GABAergic synapses. Nat. Neurosci. 15, 20–22 (2012).
Kim, J.H., Renden, R. & von Gersdorff, H. Dysmyelination of auditory afferent axons increases the jitter of action potential timing during high-frequency firing. J. Neurosci. 33, 9402–9407 (2013).
McGee, A.W., Yang, Y., Fischer, Q.S., Daw, N.W. & Strittmatter, S.M. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 309, 2222–2226 (2005).
Martina, M. & Jonas, P. Functional differences in Na+ channel gating between fast-spiking interneurones and principal neurones of rat hippocampus. J. Physiol. (Lond.) 505, 593–603 (1997).
Oxford, G.S. Some kinetic and steady-state properties of sodium channels after removal of inactivation. J. Gen. Physiol. 77, 1–22 (1981).
Sakmann, B. & Neher, E. Geometric parameters of pipettes and membrane patches. in Single-Channel Recording, 2nd edn (eds. Sakmann, B. & Neher, E.) 637–650 (Plenum Press, New York/London, 1995).
Horn, R. Statistical methods for model discrimination. Applications to gating kinetics and permeation of the acetylcholine receptor channel. Biophys. J. 51, 255–263 (1987).
Carnevale, N.T. & Hines, M.L. The Neuron Book (Cambridge University Press, Cambridge, 2006).
Hodgkin, A. The optimum density of sodium channels in an unmyelinated nerve. Phil. Trans. R. Soc. Lond. B 270, 297–300 (1975).
Aponte, Y., Lien, C.C., Reisinger, E. & Jonas, P. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J. Physiol. (Lond.) 574, 229–243 (2006).
Acknowledgements
We thank D. Debanne, J. Guzmán and R. Shigemoto for critically reading previous versions of the manuscript. We also thank F. Marr and M. Duggan for technical assistance, A. Schlögl for programming, E. Kramberger and A. Solymosi for editorial support, and M. Hines and T. Carnevale for useful suggestions. P.J. was supported by the European Union (ERC advanced grant 268548) and the Fond zur Förderung der wissenschaftlichen Forschung (FWF grant P24909-B24).
Author information
Authors and Affiliations
Contributions
H.H. performed experiments and analyzed the data. P.J. performed modeling and wrote the paper. Both authors jointly revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 High density of boutons and axon collaterals generates failures and slowing of propagation of APs in schematic models.
(a) Structure of the schematic model. Large sphere on the left represents soma (20 μm diameter), horizontal cylinder on the right is axon (1 mm length; 0.5 μm diameter), small spheres represent boutons (5 μm diameter), and vertical cylinders indicate axon collaterals (100 μm length; 0.3 μm diameter). Throughout the simulations,  in the soma was 200 pS μm-2, and
in the soma was 200 pS μm-2, and  in the axon was 1200 pS μm-2. Boutons and collaterals were introduced with equidistant spacing. Axon diameters were approximately consistent with previous electron microscopy data14. (b) APs evoked by a short current pulse at the soma (1 nA, 1 ms). Red trace, somatic AP, black traces, APs in the axon at different sites with 100 μm distance. Numbers on top indicate the number of boutons / number of collaterals. (c) Contour plot of AP propagation velocity against number of boutons and number of axon collaterals. Note failure of AP propagation in a large part of the parameter space. In both b and c, passive properties of boutons and collaterals were assumed (
in the axon was 1200 pS μm-2. Boutons and collaterals were introduced with equidistant spacing. Axon diameters were approximately consistent with previous electron microscopy data14. (b) APs evoked by a short current pulse at the soma (1 nA, 1 ms). Red trace, somatic AP, black traces, APs in the axon at different sites with 100 μm distance. Numbers on top indicate the number of boutons / number of collaterals. (c) Contour plot of AP propagation velocity against number of boutons and number of axon collaterals. Note failure of AP propagation in a large part of the parameter space. In both b and c, passive properties of boutons and collaterals were assumed ( = 0). (d, e) AP traces (d) and contour plot of AP propagation velocity (e) in the case of active boutons and collaterals (
= 0). (d, e) AP traces (d) and contour plot of AP propagation velocity (e) in the case of active boutons and collaterals ( = 1200 pS μm-2). Numbers right-adjacent to contour lines in c and e indicate AP propagation velocity (in m s−1).
= 1200 pS μm-2). Numbers right-adjacent to contour lines in c and e indicate AP propagation velocity (in m s−1).
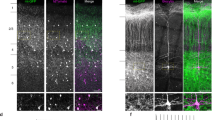
Supplementary Figure 2 Minimal myelination of BC axons in dentate gyrus of ~6-weeks-old rats.
Double immunolabeling for parvalbumin and myelin basic protein (MBP) in a 6-week-old rat. Left, parvalbumin; center, MBP; right, overlay. Note the absence of colocalization between the two markers in the granule cell layer, suggesting that BC axons are largely unmyelinated. Experiments were repeated on 5 animals, giving consistent results. White arrows indicate the outer border of the granule cell layer. Lower micrographs are expanded versions of upper images.
Supplementary Figure 3 Proximal initiation and fast, reliable propagation of APs in interneuron axons at near-physiological temperature.
(a, b) Simultaneous recording from the soma and the axon of a fast-spiking, parvalbumin-expressing BC during a long somatic current pulse. Black, somatic voltage; blue, axonal voltage. The axonal recording site was located 32 μm (a) or 153 μm (b) from the soma. Bottom traces show expanded views of the first and the 20th AP in the high-frequency train. Black traces, somatic voltage and corresponding current; blue traces, axonal voltage and corresponding current. (c) Plot of latency between somatic and axonal AP against distance of the axonal recording site from the soma for the first AP (upper graph) and the 20th AP (lower graph). Data from 19 simultaneous axon−soma recordings. Filled circles, recordings from axon varicosities; open circles, recordings from axon shafts; red line, bilinear function fit to the data points. (d) Simultaneous recording from the soma and the axon of a BC during a high-frequency train of short somatic current pulses (100 Hz, 120 stimuli; 3 ms, 1 nA). Black, somatic voltage; blue, axonal voltage. The axonal recording site was located 142 μm from the soma. (e) Plot of instantaneous somatic and axonal AP frequency during the stimulation train. The axonal AP frequency was 100 Hz, identical to the stimulation frequency. (f) Plot of ratio of number of APs in the axon over number of APs in the soma, plotted against distance of the axonal recording site. Data from 18 simultaneous axon−soma recordings. Note that the ratio was 1 in all cases, indicating high reliability of propagation.
Supplementary Figure 4 Patch area determined by capacitance measurements.
(a) Experimental configuration. The silicone elastomer ball is visible on the left, the patch pipette is located on the right. The pipette tip was gently pushed into the insulating ball, with an insertion depth of ~10 μm. (b) Difference current before and after pressing the pipette tip with the outside-out patch into the Sylgard ball. Shaded area indicates integral under the current trace. Currents were evoked by a test pulse to −100 mV from a holding potential of −50 mV. (c) Plot of patch area against pipette conductance. Data were fit by linear regression, with the parameters A(gP) = 0.08271 x gP − 0.47526, where A is patch area (μm2) and gP is pipette conductance (nS). Patch area was calculated from capacitance, assuming a specific membrane capacitance of 1 μF cm−2. The upper horizontal axis shows pipette resistance (which is the inverse of pipette conductance). Results were obtained from 7 axonal and 9 somatic outside-out patches. Somatic data are shown in black, axonal data are depicted in blue.
Supplementary Figure 5 Fast gating of Na+ channels in BC axons.
(a–c) Activation (a), inactivation (b), and deactivation time course of axonal Na+ channels (c). Outside-out patches isolated from the axon 50, 64, and 31 μm from the soma. Red curves indicate exponential functions fit to the recorded traces. The corresponding pulse protocol is shown at the bottom. (d) Plot of activation (squares), inactivation (circles), and deactivation time constants (triangles) against test pulse potential. (e) Summary bar graph of inactivation time constant at 0 mV. Bars indicate mean ± SEM, circles represent data from individual experiments (9 somatic and 11 axonal patches). Note the marked difference in the inactivation time constants between somatic and axonal Na+ channels. ** indicates P < 0.01. Somatic data are shown in black, axonal data are depicted in blue.
Supplementary Figure 6 Negative shift of voltage-dependence of Na+ channel activation and inactivation in BC axons.
(a) Na+ currents in an outside-out patch isolated from the axon 64 μm from the soma. The corresponding activation pulse protocol with increasing test pulse amplitude is shown at the bottom. (b) Na+ currents in an outside-out patch isolated from the axon 31 μm from the soma. The corresponding inactivation pulse protocol with increasing prepulse amplitude is shown at the bottom. (c) Na+ channel activation and inactivation curves. Data points indicate mean ± SEM from 6−14 patches. Data points were fit with Boltzmann functions. Note that the activation curve of axonal Na+ channels is shifted to the left in comparison to that of somatic Na+ channels. Somatic data are shown in black, axonal data are depicted in blue. Continuous and dashed lines represent Boltzmann functions fit to activation and inactivation data points, respectively.
Supplementary Figure 7 Dependence of reliability and velocity of AP propagation on axonal Na+ conductance density is independent of details of morphological properties.
(a) AP propagation in cell 2, used for the computational analysis in Fig. 4. Top, plot of reliability of AP propagation, shown as color coding of the surface of the reconstructed neuron for proximal  = distal
= distal  = 500 pS μm−2. Color scale bar indicates the reliability of propagation in the axon (inset, top). Center, plot of axon diameter against axon branch order. Black points indicate diameters of individual sections, red line represents mean. Bottom, plot of latency against distance. Note that the slope of the latency−distance relation is constant over a wide range of distances and branch orders, indicating constant AP propagation velocity. Proximal
= 500 pS μm−2. Color scale bar indicates the reliability of propagation in the axon (inset, top). Center, plot of axon diameter against axon branch order. Black points indicate diameters of individual sections, red line represents mean. Bottom, plot of latency against distance. Note that the slope of the latency−distance relation is constant over a wide range of distances and branch orders, indicating constant AP propagation velocity. Proximal  = distal
= distal  = 1000 pS μm−2. Dashed line indicates the results of linear regression for distances > 100 μm. Color scale bar indicates axon branch order (inset, top). (b) AP propagation in 5 other fully reconstructed BCs. Note that all cells except one (cell 4, center) showed absolute reliability of propagation; proximal
= 1000 pS μm−2. Dashed line indicates the results of linear regression for distances > 100 μm. Color scale bar indicates axon branch order (inset, top). (b) AP propagation in 5 other fully reconstructed BCs. Note that all cells except one (cell 4, center) showed absolute reliability of propagation; proximal  = distal
= distal  = 500 pS μm−2. (c) Structural properties of BC axons in the fully reconstructed cells. Bars indicate mean ± SEM, circles represent data from individual experiments. (d) Plot of AP propagation velocity against structural parameters of BC axons. Red points in c and d indicate cell 2 used for the simulations in Fig. 4. This cell was chosen because its axon diameter was most representative.
= 500 pS μm−2. (c) Structural properties of BC axons in the fully reconstructed cells. Bars indicate mean ± SEM, circles represent data from individual experiments. (d) Plot of AP propagation velocity against structural parameters of BC axons. Red points in c and d indicate cell 2 used for the simulations in Fig. 4. This cell was chosen because its axon diameter was most representative.
Supplementary Figure 8 Dependence of reliability and velocity of AP propagation on axon diameter and channel gating kinetics.
(a) Effects of increase in axon diameter (multiplying all diameters by a factor of 1.5). (b) Effects of decrease in axon diameter (multiplying all diameters by a factor of 0.66). (c) Effects of accelerating Na+ channel inactivation rates and K+ channel activation rates (multiplying corresponding rates by a factor of 1.73, corresponding to a 5°C temperature increase for a Q10 of 3). (d) Effects of slowing Na+ channel inactivation rates and K+ channel activation rates (multiplying corresponding rates by a factor of 0.58, corresponding to a 5°C temperature decrease for a Q10 of 3). Upper graphs, contour plots of the average slope of the reliability−distance relation as a function of proximal and distal  . Lower graphs, contour plots of AP propagation velocity as a function of proximal and distal
. Lower graphs, contour plots of AP propagation velocity as a function of proximal and distal  . Gray scale bar indicates the value of the indicated parameter (right). All simulations were performed on cell 2. Parameters of the simulations were identical to those in Fig. 4, unless specified differently.
. Gray scale bar indicates the value of the indicated parameter (right). All simulations were performed on cell 2. Parameters of the simulations were identical to those in Fig. 4, unless specified differently.
Supplementary Figure 9 Partial block of Na+ channels increases latency of evoked IPSCs at BC−GC synapses.
(a) Unitary IPSCs at BC−GC synapses under control conditions (left), in the presence of 2 nM TTX in the bath (center), and after washout (right). Upper traces, APs in BCs evoked by brief current pulses; bottom traces, average IPSCs. (b) Overlay of traces in control and 2 nM TTX (left) and control and washout (right) at different time scales. Note that 2 nM TTX reversibly increased the latency of evoked IPSCs. Upper traces are displayed at absolute current scale, lower traces are shown normalized to the same peak amplitude to facilitate comparison of synaptic latency. (c) Plot of IPSC latency against experimental time during application of 2 nM TTX (horizontal bar) from the same experiment as shown in (a, b). Each data point represents the mean of 25 consecutive IPSC latency values. (d) Summary graph of the effects of 2 nM TTX on IPSC latency. Data from 5 BC−GC pairs. Data from the same experiment were connected by lines. * indicates P < 0.05. Slight differences in the 20−80% rise time (b) were not significant (P > 0.1).
Supplementary Figure 10 A supercritical Na+ channel density in the axon ensures fast AP propagation in an experimentally recorded cell.
(a) Plot of axon diameter against axon branch order in the experimentally recorded cell. Same cell as shown in Fig. 5d, open circles. Black points indicate diameter of individual sections, red line represents mean. Note that the axon could be only partially reconstructed. (b) Simulated APs at the soma (black) and the axonal recording site (blue) under control conditions (top) and after simulated block of 43.1% of conductance (bottom).  = 600 pS μm−2 and 341 pS μm−2, respectively. Note that the latency between the two voltage signals increases, as observed experimentally. (c) Plot of reliability of AP propagation, shown as color coding of the surface of the reconstructed neuron for
= 600 pS μm−2 and 341 pS μm−2, respectively. Note that the latency between the two voltage signals increases, as observed experimentally. (c) Plot of reliability of AP propagation, shown as color coding of the surface of the reconstructed neuron for  = 500 pS μm−2. Color scale bar indicates the reliability of propagation in the axon (inset, top); dendrites are depicted in black to indicate lack of active AP propagation. Gray sphere represents the proximal region of the axon. Axonal recording pipette on an 8th order axon branch is illustrated schematically. (d) Plot of latency against distance for
= 500 pS μm−2. Color scale bar indicates the reliability of propagation in the axon (inset, top); dendrites are depicted in black to indicate lack of active AP propagation. Gray sphere represents the proximal region of the axon. Axonal recording pipette on an 8th order axon branch is illustrated schematically. (d) Plot of latency against distance for  = 500 pS μm−2. Color scale bar indicates axon branch order (inset, top). Dashed lines indicate the results of linear regression for distances > 100 μm. (e, f) Contour plots of reliability (e) and velocity (f) of AP propagation for the experimentally recorded cell. Note that the reliability of AP propagation is supported above a critical value of
= 500 pS μm−2. Color scale bar indicates axon branch order (inset, top). Dashed lines indicate the results of linear regression for distances > 100 μm. (e, f) Contour plots of reliability (e) and velocity (f) of AP propagation for the experimentally recorded cell. Note that the reliability of AP propagation is supported above a critical value of  = 200 pS μm−2, whereas the speed of propagation further increases at supercritical values of
= 200 pS μm−2, whereas the speed of propagation further increases at supercritical values of  > 200 pS μm−2. Gray scale bar indicates the value of the indicated parameter (right).
> 200 pS μm−2. Gray scale bar indicates the value of the indicated parameter (right).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 1950 kb)
Rights and permissions
About this article
Cite this article
Hu, H., Jonas, P. A supercritical density of Na+ channels ensures fast signaling in GABAergic interneuron axons. Nat Neurosci 17, 686–693 (2014). https://doi.org/10.1038/nn.3678
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3678
This article is cited by
-
Dopamine neuron degeneration in the Ventral Tegmental Area causes hippocampal hyperexcitability in experimental Alzheimer’s Disease
Molecular Psychiatry (2024)
-
Fast-spiking parvalbumin-positive interneurons in brain physiology and Alzheimer’s disease
Molecular Psychiatry (2023)
-
How connectivity rules and synaptic properties shape the efficacy of pattern separation in the entorhinal cortex–dentate gyrus–CA3 network
Nature Computational Science (2021)
-
Precise spatiotemporal control of voltage-gated sodium channels by photocaged saxitoxin
Nature Communications (2021)
-
An axon-specific expression of HCN channels catalyzes fast action potential signaling in GABAergic interneurons
Nature Communications (2020)