Abstract
The role of voltage-gated Ca2+ channels (VGCCs) in spontaneous miniature neurotransmitter release is incompletely understood. We found that stochastic opening of P/Q-, N- and R-type VGCCs accounts for ∼50% of all spontaneous glutamate release at rat cultured hippocampal synapses, and that R-type channels have a far greater role in spontaneous than in action potential–evoked exocytosis. VGCC-dependent miniature neurotransmitter release (minis) showed similar sensitivity to presynaptic Ca2+ chelation as evoked release, arguing for direct triggering of spontaneous release by transient spatially localized Ca2+ domains. Experimentally constrained three-dimensional diffusion modeling of Ca2+ influx–exocytosis coupling was consistent with clustered distribution of VGCCs in the active zone of small hippocampal synapses and revealed that spontaneous VGCCs openings can account for the experimentally observed VGCC-dependent minis, although single channel openings triggered release with low probability. Uncorrelated stochastic VGCC opening is therefore a major trigger for spontaneous glutamate release, with differential roles for distinct channel subtypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sutton, M.A., Wall, N.R., Aakalu, G.N. & Schuman, E.M. Regulation of dendritic protein synthesis by miniature synaptic events. Science 304, 1979–1983 (2004).
McKinney, R.A., Capogna, M., Durr, R., Gahwiler, B.H. & Thompson, S.M. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat. Neurosci. 2, 44–49 (1999).
Groffen, A.J. et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science 327, 1614–1618 (2010).
Xu, J., Pang, Z.P., Shin, O.H. & Sudhof, T.C. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat. Neurosci. 12, 759–766 (2009).
Eggermann, E., Bucurenciu, I., Goswami, S.P. & Jonas, P. Nanodomain coupling between Ca(2+) channels and sensors of exocytosis at fast mammalian synapses. Nat. Rev. Neurosci. 13, 7–21 (2012).
Williams, C. et al. Coactivation of multiple tightly coupled calcium channels triggers spontaneous release of GABA. Nat. Neurosci. 15, 1195–1197 (2012).
Goswami, S.P., Bucurenciu, I. & Jonas, P. Miniature IPSCs in hippocampal granule cells are triggered by voltage-gated Ca2+ channels via microdomain coupling. J. Neurosci. 32, 14294–14304 (2012).
Vyleta, N.P. & Smith, S.M. Spontaneous glutamate release is independent of calcium influx and tonically activated by the calcium-sensing receptor. J. Neurosci. 31, 4593–4606 (2011).
Scanziani, M., Capogna, M., Gahwiler, B.H. & Thompson, S.M. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron 9, 919–927 (1992).
Wu, L.G. & Saggau, P. Pharmacological identification of two types of presynaptic voltage-dependent calcium channels at CA3–CA1 synapses of the hippocampus. J. Neurosci. 14, 5613–5622 (1994).
Sheng, J. et al. Calcium-channel number critically influences synaptic strength and plasticity at the active zone. Nat. Neurosci. 15, 998–1006 (2012).
Li, L., Bischofberger, J. & Jonas, P. Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J. Neurosci. 27, 13420–13429 (2007).
Reid, C.A., Bekkers, J.M. & Clements, J.D. N- and P/Q-type Ca2+ channels mediate transmitter release with a similar cooperativity at rat hippocampal autapses. J. Neurosci. 18, 2849–2855 (1998).
Mintz, I.M., Sabatini, B.L. & Regehr, W.G. Calcium control of transmitter release at a cerebellar synapse. Neuron 15, 675–688 (1995).
Holderith, N. et al. Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat. Neurosci. 15, 988–997 (2012).
Soong, T.W. et al. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science 260, 1133–1136 (1993).
Regehr, W.G. & Atluri, P.P. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys. J. 68, 2156–2170 (1995).
Xu, B. et al. Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS ONE 6, e19052 (2011).
Lou, X., Scheuss, V. & Schneggenburger, R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature 435, 497–501 (2005).
Neher, E. Usefulness and limitations of linear approximations to the understanding of Ca2+ signals. Cell Calcium 24, 345–357 (1998).
Atluri, P.P. & Regehr, W.G. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J. Neurosci. 16, 5661–5671 (1996).
Ohana, O. & Sakmann, B. Transmitter release modulation in nerve terminals of rat neocortical pyramidal cells by intracellular calcium buffers. J. Physiol. (Lond.) 513, 135–148 (1998).
Bucurenciu, I., Kulik, A., Schwaller, B., Frotscher, M. & Jonas, P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron 57, 536–545 (2008).
Murthy, V.N., Schikorski, T., Stevens, C.F. & Zhu, Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron 32, 673–682 (2001).
Ermolyuk, Y.S. et al. Independent regulation of basal neurotransmitter release efficacy by variable Ca2+ influx and bouton size at small central synapses. PLoS Biol. 10, e1001396 (2012).
Gaffield, M.A. & Betz, W.J. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nat. Protoc. 1, 2916–2921 (2006).
Hoppa, M.B., Lana, B., Margas, W., Dolphin, A.C. & Ryan, T.A. Alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature 486, 122–125 (2012).
Meinrenken, C.J., Borst, J.G. & Sakmann, B. Calcium secretion coupling at calyx of held governed by nonuniform channel-vesicle topography. J. Neurosci. 22, 1648–1667 (2002).
Scimemi, A. & Diamond, J.S. The number and organization of Ca2+ channels in the active zone shapes neurotransmitter release from Schaffer collateral synapses. J. Neurosci. 32, 18157–18176 (2012).
Ariel, P. & Ryan, T.A. Optical mapping of release properties in synapses. Front. Neural Circuits 4, 18 (2010).
Rozov, A., Burnashev, N., Sakmann, B. & Neher, E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J. Physiol. (Lond.) 531, 807–826 (2001).
Schikorski, T. & Stevens, C.F. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J. Neurosci. 17, 5858–5867 (1997).
Novak, P. et al. Nanoscale-targeted patch-clamp recordings of functional presynaptic ion channels. Neuron 79, 1067–1077 (2013).
Shepherd, G.M. & Harris, K.M. Three-dimensional structure and composition of CA3→CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. 18, 8300–8310 (1998).
Siksou, L. et al. Three-dimensional architecture of presynaptic terminal cytomatrix. J. Neurosci. 27, 6868–6877 (2007).
Bucurenciu, I., Bischofberger, J. & Jonas, P. A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nat. Neurosci. 13, 19–21 (2010).
Matveev, V., Bertram, R. & Sherman, A. Calcium cooperativity of exocytosis as a measure of Ca2+ channel domain overlap. Brain Res. 1398, 126–138 (2011).
Weber, A.M. et al. N-type Ca2+ channels carry the largest current: implications for nanodomains and transmitter release. Nat. Neurosci. 13, 1348–1350 (2010).
Atasoy, D. et al. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J. Neurosci. 28, 10151–10166 (2008).
Geppert, M. et al. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79, 717–727 (1994).
Murthy, V.N. & Stevens, C.F. Reversal of synaptic vesicle docking at central synapses. Nat. Neurosci. 2, 503–507 (1999).
Sharma, G. & Vijayaraghavan, S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron 38, 929–939 (2003).
Emptage, N.J., Reid, C.A. & Fine, A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron 29, 197–208 (2001).
Mochida, S. et al. Requirement for the synaptic protein interaction site for reconstitution of synaptic transmission by P/Q-type calcium channels. Proc. Natl. Acad. Sci. USA 100, 2819–2824 (2003).
Kaeser, P.S. et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144, 282–295 (2011).
Bao, J., Li, J.J. & Perl, E.R. Differences in Ca2+ channels governing generation of miniature and evoked excitatory synaptic currents in spinal laminae I and II. J. Neurosci. 18, 8740–8750 (1998).
Pavlov, I., Scimemi, A., Savtchenko, L., Kullmann, D.M. & Walker, M.C. Ih-mediated depolarization enhances the temporal precision of neuronal integration. Nat. Commun. 2, 199 (2011).
Banke, T.G. & McBain, C.J. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J. Neurosci. 26, 11720–11725 (2006).
Ruiz, A., Campanac, E., Scott, R.S., Rusakov, D.A. & Kullmann, D.M. Presynaptic GABAA receptors enhance transmission and LTP induction at hippocampal mossy fiber synapses. Nat. Neurosci. 13, 431–438 (2010).
Alle, H. & Geiger, J.R. Analog signalling in mammalian cortical axons. Curr. Opin. Neurobiol. 18, 314–320 (2008).
Dreyfus, F.M. et al. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J. Neurosci. 30, 99–109 (2010).
Maravall, M., Mainen, Z.F., Sabatini, B.L. & Svoboda, K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys. J. 78, 2655–2667 (2000).
Benson, D.L., Watkins, F.H., Steward, O. & Banker, G. Characterization of GABAergic neurons in hippocampal cell cultures. J. Neurocytol. 23, 279–295 (1994).
Hines, M.L. & Carnevale, N.T. The NEURON simulation environment. Neural Comput. 9, 1179–1209 (1997).
Sasaki, T., Matsuki, N. & Ikegaya, Y. Action-potential modulation during axonal conduction. Science 331, 599–601 (2011).
Borst, J.G., Helmchen, F. & Sakmann, B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J. Physiol. (Lond.) 489, 825–840 (1995).
Scott, R. & Rusakov, D.A. Main determinants of presynaptic Ca2+ dynamics at individual mossy fiber-CA3 pyramidal cell synapses. J. Neurosci. 26, 7071–7081 (2006).
Faas, G.C., Raghavachari, S., Lisman, J.E. & Mody, I. Calmodulin as a direct detector of Ca2+ signals. Nat. Neurosci. 14, 301–304 (2011).
Nägerl, U.V., Novo, D., Mody, I. & Vergara, J.L. Binding kinetics of calbindin-D(28k) determined by flash photolysis of caged Ca2+. Biophys. J. 79, 3009–3018 (2000).
Xia, Z. & Storm, D.R. The role of calmodulin as a signal integrator for synaptic plasticity. Nat. Rev. Neurosci. 6, 267–276 (2005).
Acknowledgements
We are grateful to L. Savtchenko for help with stochastic VGCC modeling, to P. Volynsky for help with Monte-Carlo simulations of VGCC distributions in the active zone, to M. Cano for help with neuronal cultures, to V. Uebele (Merck) for the gift of TTA-P2, and to Y. Ushkaryov, D. Rusakov, C. Henneberger and M. Walker for critical reading of the manuscript. The study was supported by the Medical Research Council, the Wellcome Trust, the Biotechnology and Biological Sciences Research Council, the German Research Foundation, the Brain Research Trust, the European Research Council, the Special Trustees of the University College London Hospitals National Health Service Foundation Trust, Epilepsy Research UK, and The Worshipful Company of Pewterers.
Author information
Authors and Affiliations
Contributions
Y.S.E., F.G.A., R.S. and I.Y.P. performed the experiments. Y.S.E., F.G.A., R.S., I.Y.P. and K.E.V. analyzed the data. Y.T. and K.E.V. performed the computational modeling. D.M.K. and K.E.V. conceived and designed the experiments. D.M.K., Y.T. and K.E.V. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Membrane depolarization with elevated extracellular 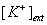 leads to an increase in VGCC-dependent miniature release.
leads to an increase in VGCC-dependent miniature release.
(a, b) Time course of mEPSC frequency changes after application of 20 mM  followed by simultaneous blockade of P/Q-, N-, and R-type VGCCs with ω-Aga, ω-Ctx and SNX. (a) mEPSC traces from a representative experiment and (b) average time course in N=7 cells (mean ± s.e.m). The integration periods used to determine the effects of 20mM
followed by simultaneous blockade of P/Q-, N-, and R-type VGCCs with ω-Aga, ω-Ctx and SNX. (a) mEPSC traces from a representative experiment and (b) average time course in N=7 cells (mean ± s.e.m). The integration periods used to determine the effects of 20mM  and VGCC blockers on average mEPSC frequency are indicated by black circles. * P < 0.05, Wilcoxon signed rank test for paired data.
and VGCC blockers on average mEPSC frequency are indicated by black circles. * P < 0.05, Wilcoxon signed rank test for paired data.
Supplementary Figure 2 Action potential-evoked currents mediated by single P/Q-, N-, and R-type VGCCs simulated using the six-state VGCC gating model.
Results of 20 representative simulations for each channel subtype (blue, P/Q-; green, N-; and brown, R-type channels). Action potential waveform is shown on the top left. Average current time courses including failures are shown at bottom (bold traces). The probability that an individual channel opens during an action potential (estimated from 500 simulations) was: Popen_P/Q = 0.50, Popen_N = 0.40, and Popen_R = 0.32.
Supplementary Figure 3 Clustered model, additional analysis: VGCC cooperativity in triggering action potential-evoked release varies with docked vesicle-VGCC cluster distance.
(a) Detailed schematic representation of the active zone for the Clustered model illustrated in Fig. 6c. VGCC positions, subtypes, and their open or closed status during the simulated action potential are specified as indicated in the insert on the right. In this model implementation 12 out of 32 VGCCs opened during the action potential (5 channels opened in the cluster located to the left side of the active zone and 7 channels opened in the cluster located to the right side of the active zone). (b) Spatiotemporal  profile during the simulated action potential within a 5 nm thick plane immediately above the active zone. Top, action potential waveform; bottom, color-coded
profile during the simulated action potential within a 5 nm thick plane immediately above the active zone. Top, action potential waveform; bottom, color-coded  map at different time points as indicated. Note that at the late action potential repolarization stage when Ca2+ currents through individual VGCCs are maximal (i.e. at 0.45 ms and 0.6 ms) Ca2+ influx in each channel cluster is mediated only by 1 or 2 VGCCs. For example, the red circle in (a) and (b) highlights two VGCCs (of N-type) that contribute most of the action potential-evoked Ca2+ current in the left channel cluster. Scale bar 50 nm. (c, d) To determine the relative contributions of the two channels highlighted in (a) and (b) to triggering release of vesicles V1 - V4 we either selectively switched them off (c, top) or left them active and switched off all other channels (c, bottom). (d) Average
map at different time points as indicated. Note that at the late action potential repolarization stage when Ca2+ currents through individual VGCCs are maximal (i.e. at 0.45 ms and 0.6 ms) Ca2+ influx in each channel cluster is mediated only by 1 or 2 VGCCs. For example, the red circle in (a) and (b) highlights two VGCCs (of N-type) that contribute most of the action potential-evoked Ca2+ current in the left channel cluster. Scale bar 50 nm. (c, d) To determine the relative contributions of the two channels highlighted in (a) and (b) to triggering release of vesicles V1 - V4 we either selectively switched them off (c, top) or left them active and switched off all other channels (c, bottom). (d) Average  concentration transients at vesicular release sensors and corresponding vesicle fusion probabilities Pv (shown above) for each vesicle in the active zone. Black traces, original control simulation; red traces, the two highlighted channels 'switched off', blue traces, all but the two highlighted channels 'switched off'. The results of the above simulations show that: (1) The two highlighted channels contributed most of the action potential-evoked
concentration transients at vesicular release sensors and corresponding vesicle fusion probabilities Pv (shown above) for each vesicle in the active zone. Black traces, original control simulation; red traces, the two highlighted channels 'switched off', blue traces, all but the two highlighted channels 'switched off'. The results of the above simulations show that: (1) The two highlighted channels contributed most of the action potential-evoked  concentration transients at release sensors of vesicle V1, which was located in the immediate vicinity of these two VGCCs (30 and 40 nm). Switching these two channels off led to a ∼22-fold reduction of the vesicle fusion probability Pv, (from 0.22 to 0.01). In contrast, switching off all other VGCCs in the active zone led to only a 2.4-fold reduction in Pv of Vesicle V1. Thus, in this model realization fusion of vesicle V1 is mainly controlled by the two highlighted channels. (2) The two highlighted channels had a minimal effect on
concentration transients at release sensors of vesicle V1, which was located in the immediate vicinity of these two VGCCs (30 and 40 nm). Switching these two channels off led to a ∼22-fold reduction of the vesicle fusion probability Pv, (from 0.22 to 0.01). In contrast, switching off all other VGCCs in the active zone led to only a 2.4-fold reduction in Pv of Vesicle V1. Thus, in this model realization fusion of vesicle V1 is mainly controlled by the two highlighted channels. (2) The two highlighted channels had a minimal effect on  concentration transients at the release sensors of vesicle V4, and as a consequence on Pv for this vesicle, which was located close (~30 nm) to the other VGCC cluster. This illustrates that action potential-evoked fusion of vesicles located in the immediate vicinity of VGCC clusters is mainly controlled by channels from the nearest cluster. (3) Finally, for vesicles that were further away from the VGCC clusters, and therefore had lower Pv (vesicles V2 and V4), action potential-evoked fusion was jointly controlled by VGCCs from both clusters.
concentration transients at the release sensors of vesicle V4, and as a consequence on Pv for this vesicle, which was located close (~30 nm) to the other VGCC cluster. This illustrates that action potential-evoked fusion of vesicles located in the immediate vicinity of VGCC clusters is mainly controlled by channels from the nearest cluster. (3) Finally, for vesicles that were further away from the VGCC clusters, and therefore had lower Pv (vesicles V2 and V4), action potential-evoked fusion was jointly controlled by VGCCs from both clusters.
Supplementary Figure 4 Dependency of stochastic VGCC openings on Vrest.
Representative traces of Ca2+ currents simulated using the six-state VGCC gating model at different Vrest in a typical active zone containing 15 P/Q-, 16 N-, and 2 R-type VGCCs. These simulations (in total 200 s for each Vrest value) showed that within the physiological Vrest range (from –55 to –80 mV) the probability of coincident opening of more than one channel in the active zone is lower than 0.002.
Supplementary Figure 5 Modeling of miniature glutamate release triggered by Ca2+ release from intracellular stores. Comparison of 0.5 mM BAPTA and 5 mM EGTA effects.
(a, b) To model the small (∼1 μM) elevations of presynaptic  from
from  = 50 nM, as might occur during Ca2+ release from intracellular stores (e.g. refs. 42,43), we used a single-compartment model of presynaptic Ca2+ dynamics (see Online Methods for details). (a) Ca2+ influx into the cytosol from the stores was approximated by a Gaussian function
= 50 nM, as might occur during Ca2+ release from intracellular stores (e.g. refs. 42,43), we used a single-compartment model of presynaptic Ca2+ dynamics (see Online Methods for details). (a) Ca2+ influx into the cytosol from the stores was approximated by a Gaussian function  with a maximal rate
with a maximal rate  = 1 μM ms-1 (~100 fold slower than that during an action potential) and with a characteristic duration σ = 2 s. (b) Resulting global presynaptic
= 1 μM ms-1 (~100 fold slower than that during an action potential) and with a characteristic duration σ = 2 s. (b) Resulting global presynaptic  concentration transients predicted by the non-stationary model in 'Control' conditions (black trace) and in the presence of 0.5 mM BAPTA (red trace) and 5 mM EGTA (blue trace). Note that, in contrast to 5 mM EGTA, 0.5 mM BAPTA had only minor effect on the presynaptic
concentration transients predicted by the non-stationary model in 'Control' conditions (black trace) and in the presence of 0.5 mM BAPTA (red trace) and 5 mM EGTA (blue trace). Note that, in contrast to 5 mM EGTA, 0.5 mM BAPTA had only minor effect on the presynaptic  transient. (c) Vesicular release rates and (d) fusion probabilities Pv, corresponding to
transient. (c) Vesicular release rates and (d) fusion probabilities Pv, corresponding to  transients shown in (b). These were calculated using the same allosteric model as used to model VGCC-dependent glutamate release (Fig. 6a). Consistent with the effect of Ca2+ chelators on the global presynaptic
transients shown in (b). These were calculated using the same allosteric model as used to model VGCC-dependent glutamate release (Fig. 6a). Consistent with the effect of Ca2+ chelators on the global presynaptic  transients, 5 mM EGTA was much more efficient in inhibiting of store-mediated miniature release (by ~88%) than 0.5 mM BAPTA (by ~13%).
transients, 5 mM EGTA was much more efficient in inhibiting of store-mediated miniature release (by ~88%) than 0.5 mM BAPTA (by ~13%).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 866 kb)
Rights and permissions
About this article
Cite this article
Ermolyuk, Y., Alder, F., Surges, R. et al. Differential triggering of spontaneous glutamate release by P/Q-, N- and R-type Ca2+ channels. Nat Neurosci 16, 1754–1763 (2013). https://doi.org/10.1038/nn.3563
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3563
This article is cited by
-
The Mechanism of α2 adrenoreceptor-dependent Modulation of Neurotransmitter Release at the Neuromuscular Junctions
Neurochemical Research (2024)
-
The release of inhibition model reproduces kinetics and plasticity of neurotransmitter release in central synapses
Communications Biology (2023)
-
Identification of Dp140 and α1-syntrophin as novel molecular interactors of the neuronal CaV2.1 channel
Pflügers Archiv - European Journal of Physiology (2023)
-
Asynchronous glutamate release is enhanced in low release efficacy synapses and dispersed across the active zone
Nature Communications (2022)
-
Asynchronous release sites align with NMDA receptors in mouse hippocampal synapses
Nature Communications (2021)




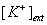 leads to an increase in VGCC-dependent miniature release.
leads to an increase in VGCC-dependent miniature release.