Abstract
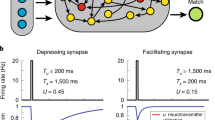
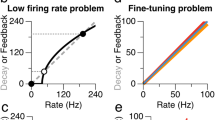
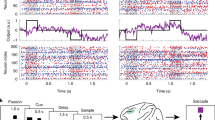
Persistent neural activity in the absence of a stimulus has been identified as a neural correlate of working memory, but how such activity is maintained by neocortical circuits remains unknown. We used a computational approach to show that the inhibitory and excitatory microcircuitry of neocortical memory-storing regions is sufficient to implement a corrective feedback mechanism that enables persistent activity to be maintained stably for prolonged durations. When recurrent excitatory and inhibitory inputs to memory neurons were balanced in strength and offset in time, drifts in activity triggered a corrective signal that counteracted memory decay. Circuits containing this mechanism temporally integrated their inputs, generated the irregular neural firing observed during persistent activity and were robust against common perturbations that severely disrupted previous models of short-term memory storage. These results reveal a mechanism for the accumulation and storage of memories in neocortical circuits based on principles of corrective negative feedback that are widely used in engineering applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jonides, J. et al. The mind and brain of short-term memory. Annu. Rev. Psychol. 59, 193–224 (2008).
Fuster, J.M. & Alexander, G.E. Neuron activity related to short-term memory. Science 173, 652–654 (1971).
Major, G. & Tank, D. Persistent neural activity: prevalence and mechanisms. Curr. Opin. Neurobiol. 14, 675–684 (2004).
Durstewitz, D., Seamans, J.K. & Sejnowski, T.J. Neurocomputational models of working memory. Nat. Neurosci. 3, 1184–1191 (2000).
Wang, X.J. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 24, 455–463 (2001).
Brody, C.D., Romo, R. & Kepecs, A. Basic mechanisms for graded persistent activity: discrete attractors, continuous attractors and dynamic representations. Curr. Opin. Neurobiol. 13, 204–211 (2003).
Seung, H.S. How the brain keeps the eyes still. Proc. Natl. Acad. Sci. USA 93, 13339–13344 (1996).
Machens, C.K., Romo, R. & Brody, C.D. Flexible control of mutual inhibition: a neural model of two-interval discrimination. Science 307, 1121–1124 (2005).
Wang, X.J. Decision making in recurrent neuronal circuits. Neuron 60, 215–234 (2008).
Haider, B. & McCormick, D.A. Rapid neocortical dynamics: cellular and network mechanisms. Neuron 62, 171–189 (2009).
Wang, H., Stradtman, G.G., Wang, X.J. & Gao, W.J. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc. Natl. Acad. Sci. USA 105, 16791–16796 (2008).
Wang, H.X. & Gao, W.J. Cell type–specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology 34, 2028–2040 (2009).
Rotaru, D.C., Yoshino, H., Lewis, D.A., Ermentrout, G.B. & Gonzalez-Burgos, G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J. Neurosci. 31, 142–156 (2011).
Wang, M. et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77, 736–749 (2013).
Softky, W.R. & Koch, C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J. Neurosci. 13, 334–350 (1993).
Compte, A. et al. Temporally irregular mnemonic persistent activity in prefrontal neurons of monkeys during a delayed response task. J. Neurophysiol. 90, 3441–3454 (2003).
Haider, B., Duque, A., Hasenstaub, A.R. & McCormick, D.A. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J. Neurosci. 26, 4535–4545 (2006).
Shu, Y., Hasenstaub, A. & McCormick, D.A. Turning on and off recurrent balanced cortical activity. Nature 423, 288–293 (2003).
Murphy, B.K. & Miller, K.D. Balanced amplification: a new mechanism of selective amplification of neural activity patterns. Neuron 61, 635–648 (2009).
Lisman, J.E., Fellous, J.M. & Wang, X.J. A role for NMDA-receptor channels in working memory. Nat. Neurosci. 1, 273–275 (1998).
Wang, X.J. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J. Neurosci. 19, 9587–9603 (1999).
Koulakov, A.A., Raghavachari, S., Kepecs, A. & Lisman, J.E. Model for a robust neural integrator. Nat. Neurosci. 5, 775–782 (2002).
Goldman, M.S., Levine, J.H., Major, G., Tank, D.W. & Seung, H.S. Robust persistent neural activity in a model integrator with multiple hysteretic dendrites per neuron. Cereb. Cortex 13, 1185–1195 (2003).
Nikitchenko, M. & Koulakov, A. Neural integrator: a sandpile model. Neural Comput. 20, 2379–2417 (2008).
Shen, L. Neural integration by short term potentiation. Biol. Cybern. 61, 319–325 (1989).
Wang, Y. et al. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat. Neurosci. 9, 534–542 (2006).
Mongillo, G., Barak, O. & Tsodyks, M. Synaptic theory of working memory. Science 319, 1543–1546 (2008).
Barbieri, F. & Brunel, N. Can attractor network models account for the statistics of firing during persistent activity in prefrontal cortex? Front. Neurosci. 2, 114–122 (2008).
Vogels, T.P., Rajan, K. & Abbott, L.F. Neural network dynamics. Annu. Rev. Neurosci. 28, 357–376 (2005).
van Vreeswijk, C. & Sompolinsky, H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science 274, 1724–1726 (1996).
Knill, D.C. & Pouget, A. The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci. 27, 712–719 (2004).
Boerlin, M. & Deneve, S. Spike-based population coding and working memory. PLoS Comput. Biol. 7, e1001080 (2011).
Romo, R., Brody, C.D., Hernandez, A. & Lemus, L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature 399, 470–473 (1999).
Roitman, J.D. & Shadlen, M.N. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci. 22, 9475–9489 (2002).
Robinson, D.A. Integrating with neurons. Annu. Rev. Neurosci. 12, 33–45 (1989).
Cannon, S.C., Robinson, D.A. & Shamma, S. A proposed neural network for the integrator of the oculomotor system. Biol. Cybern. 49, 127–136 (1983).
Shadlen, M.N., Britten, K.H., Newsome, W.T. & Movshon, J.A. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J. Neurosci. 16, 1486–1510 (1996).
Shadlen, M.N. & Newsome, W.T. Noise, neural codes and cortical organization. Curr. Opin. Neurobiol. 4, 569–579 (1994).
Destexhe, A., Rudolph, M. & Pare, D. The high-conductance state of neocortical neurons in vivo. Nat. Rev. Neurosci. 4, 739–751 (2003).
Renart, A., Moreno-Bote, R., Wang, X.J. & Parga, N. Mean-driven and fluctuation-driven persistent activity in recurrent networks. Neural Comput. 19, 1–46 (2007).
Roudi, Y. & Latham, P.E. A balanced memory network. PLoS Comput. Biol. 3, 1679–1700 (2007).
Major, G., Polsky, A., Denk, W., Schiller, J. & Tank, D.W. Spatiotemporally graded NMDA spike/plateau potentials in basal dendrites of neocortical pyramidal neurons. J. Neurophysiol. 99, 2584–2601 (2008).
Liu, G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat. Neurosci. 7, 373–379 (2004).
Tao, H.W. & Poo, M.M. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron 45, 829–836 (2005).
Vogels, T.P., Sprekeler, H., Zenke, F., Clopath, C. & Gerstner, W. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 334, 1569–1573 (2011).
Xie, X. & Seung, H.S. Spike-based learning rules and stabilization of persistent neural activity. in Advances in Neural Information Processing Systems Vol. 12 (eds. Solla, S.A., Leen, T.K. & Müller, K.-R.) 199–205 (2000).
Csete, M.E. & Doyle, J.C. Reverse engineering of biological complexity. Science 295, 1664–1669 (2002).
Ganguli, S. et al. One-dimensional dynamics of attention and decision making in LIP. Neuron 58, 15–25 (2008).
Coyle, J.T., Tsai, G. & Goff, D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann. NY Acad. Sci. 1003, 318–327 (2003).
Wilson, H.R. Spikes, Decisions and Actions (Oxford University Press, 1999).
McCormick, D.A., Connors, B.W., Lighthall, J.W. & Prince, D.A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J. Neurophysiol. 54, 782–806 (1985).
Salin, P.A. & Prince, D.A. Spontaneous GABAA receptor–mediated inhibitory currents in adult rat somatosensory cortex. J. Neurophysiol. 75, 1573–1588 (1996).
Xiang, Z., Huguenard, J.R. & Prince, D.A. GABAA receptor-mediated currents in interneurons and pyramidal cells of rat visual cortex. J. Physiol. (Lond.) 506, 715–730 (1998).
Hansel, D., Mato, G., Meunier, C. & Neltner, L. On numerical simulations of integrate-and-fire neural networks. Neural Comput. 10, 467–483 (1998).
Acknowledgements
We thank D. Fisher for valuable discussions and E. Aksay, K. Britten, N. Brunel, D. Butts, J. Ditterich, R. Froemke, A. Goddard, D. Kastner, B. Lankow, S. Luck, B. Mulloney, J. Raymond, J. Rinzel and M. Usrey for valuable discussions and feedback on the manuscript. We thank A. Lerchner for providing code for our initial simulations of spiking network models. This research was supported by US National Institutes of Health grants R01 MH069726 and R01 MH065034, a Sloan Foundation fellowship, and a University of California Davis Ophthalmology Research to Prevent Blindness grant.
Author information
Authors and Affiliations
Contributions
S.L. and M.S.G. designed the study, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Modeling (PDF 8159 kb)
Rights and permissions
About this article
Cite this article
Lim, S., Goldman, M. Balanced cortical microcircuitry for maintaining information in working memory. Nat Neurosci 16, 1306–1314 (2013). https://doi.org/10.1038/nn.3492
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3492
This article is cited by
-
Synaptic wiring motifs in posterior parietal cortex support decision-making
Nature (2024)
-
On the physiological and structural contributors to the overall balance of excitation and inhibition in local cortical networks
Journal of Computational Neuroscience (2024)
-
Choice selective inhibition drives stability and competition in decision circuits
Nature Communications (2023)
-
Latent Space Exploration and Functionalization of a Gated Working Memory Model Using Conceptors
Cognitive Computation (2023)
-
Robust working memory in a two-dimensional continuous attractor network
Cognitive Neurodynamics (2023)