Abstract
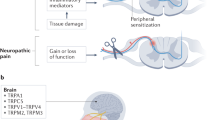
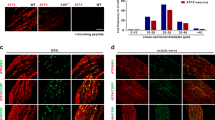
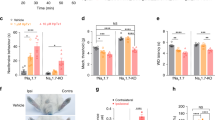
TMEM16C belongs to the TMEM16 family, which includes the Ca2+-activated Cl− channels TMEM16A and TMEM16B and a small-conductance, Ca2+-activated, nonselective cation channel (SCAN), TMEM16F. We found that in rat dorsal root ganglia (DRG) TMEM16C was expressed mainly in the IB4-positive, non-peptidergic nociceptors that also express the sodium-activated potassium (KNa) channel Slack. Together these channel proteins promote KNa channel activity and dampen neuronal excitability. DRG from TMEM16C knockout rats had diminished Slack expression, broadened action potentials and increased excitability. Moreover, the TMEM16C knockout rats, as well as rats with Slack knockdown by intrathecal injection of short interfering RNA, exhibited increased thermal and mechanical sensitivity. Experiments involving heterologous expression in HEK293 cells further showed that TMEM16C modulated the single-channel activity of Slack channels and increased its sodium sensitivity. Our study thus reveals that TMEM16C enhances KNa channel activity in DRG neurons and regulates the processing of pain messages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yang, Y.D. et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215 (2008).
Schroeder, B.C., Cheng, T., Jan, Y.N. & Jan, L.Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134, 1019–1029 (2008).
Caputo, A. et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594 (2008).
Huang, F. et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc. Natl. Acad. Sci. USA 109, 16354–16359 (2012).
Huang, F. et al. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc. Natl. Acad. Sci. USA 106, 21413–21418 (2009).
Cho, H. et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 15, 1015–1021 (2012).
Stöhr, H. et al. TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J. Neurosci. 29, 6809–6818 (2009).
Huang, W.C. et al. Calcium-activated chloride channels (CaCCs) regulate action potential and synaptic response in hippocampal neurons. Neuron 74, 179–192 (2012).
Billig, G.M., Pal, B., Fidzinski, P. & Jentsch, T.J. Ca2+-activated Cl− currents are dispensable for olfaction. Nat. Neurosci. 14, 763–769 (2011).
Yang, H. et al. TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 151, 111–122 (2012).
Suzuki, J., Umeda, M., Sims, P.J. & Nagata, S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468, 834–838 (2010).
Oldham, M.C., Horvath, S. & Geschwind, D.H. Conservation and evolution of gene coexpression networks in human and chimpanzee brains. Proc. Natl. Acad. Sci. USA 103, 17973–17978 (2006).
Briones, N. & Dinu, V. Data mining of high density genomic variant data for prediction of Alzheimer's disease risk. BMC Med. Genet. 13, 7 (2012).
Charlesworth, G. et al. Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis. Am. J. Hum. Genet. 91, 1041–1050 (2012).
Basbaum, A.I., Bautista, D.M., Scherrer, G. & Julius, D. Cellular and molecular mechanisms of pain. Cell 139, 267–284 (2009).
Dhaka, A. et al. TRPM8 is required for cold sensation in mice. Neuron 54, 371–378 (2007).
Davis, J.B. et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183–187 (2000).
Snider, W.D. & McMahon, S.B. Tackling pain at the source: new ideas about nociceptors. Neuron 20, 629–632 (1998).
Dong, X., Han, S., Zylka, M.J., Simon, M.I. & Anderson, D.J. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106, 619–632 (2001).
Julius, D. & Basbaum, A.I. Molecular mechanisms of nociception. Nature 413, 203–210 (2001).
Noël, J. et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 28, 1308–1318 (2009).
Vivancos, G.G. et al. An electronic pressure-meter nociception paw test for rats. Braz. J. Med. Biol. Res. 37, 391–399 (2004).
Stucky, C.L. & Lewin, G.R. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J. Neurosci. 19, 6497–6505 (1999).
Tian, Y., Schreiber, R. & Kunzelmann, K. Anoctamins are a family of Ca2+ activated Cl− channels. J. Cell Sci. 125, 4991–4998 (2012).
Duran, C., Qu, Z., Osunkoya, A.O., Cui, Y. & Hartzell, H.C. ANOs 3–7 in the anoctamin/Tmem16 Cl− channel family are intracellular proteins. Am. J. Physiol. Cell Physiol. 302, C482–C493 (2012).
Leffler, A., Herzog, R.I., Dib-Hajj, S.D., Waxman, S.G. & Cummins, T.R. Pharmacological properties of neuronal TTX-resistant sodium channels and the role of a critical serine pore residue. Pflugers Arch. 451, 454–463 (2005).
Kuo, C.C., Lin, T.J. & Hsieh, C.P. Effect of Na+ flow on Cd2+ block of tetrodotoxin-resistant Na+ channels. J. Gen. Physiol. 120, 159–172 (2002).
Rush, A.M., Cummins, T.R. & Waxman, S.G. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J. Physiol. (Lond.) 579, 1–14 (2007).
Huang, Y., Quayle, J.M., Worley, J.F., Standen, N.B. & Nelson, M.T. External cadmium and internal calcium block of single calcium channels in smooth muscle cells from rabbit mesenteric artery. Biophys. J. 56, 1023–1028 (1989).
Ryglewski, S., Pflueger, H.J. & Duch, C. Expanding the neuron's calcium signaling repertoire: intracellular calcium release via voltage-induced PLC and IP3R activation. PLoS Biol. 5, e66 (2007).
Catterall, W.A., Perez-Reyes, E., Snutch, T.P. & Striessnig, J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 57, 411–425 (2005).
Blair, N.T. & Bean, B.P. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J. Neurosci. 22, 10277–10290 (2002).
Tamsett, T.J., Picchione, K.E. & Bhattacharjee, A. NAD+ activates KNa channels in dorsal root ganglion neurons. J. Neurosci. 29, 5127–5134 (2009).
Nanou, E. et al. Na+-mediated coupling between AMPA receptors and KNa channels shapes synaptic transmission. Proc. Natl. Acad. Sci. USA 105, 20941–20946 (2008).
Bhattacharjee, A. & Kaczmarek, L.K. For K+ channels, Na+ is the new Ca2+. Trends Neurosci. 28, 422–428 (2005).
Yuan, A. et al. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron 37, 765–773 (2003).
Kamiyama, D. & Huang, B. Development in the STORM. Dev. Cell 23, 1103–1110 (2012).
Heninger, A.K. & Buchholz, F. Production of endoribonuclease-prepared short interfering RNAs (esiRNAs) for specific and effective gene silencing in mammalian cells. CSH Protoc. 2007, pdb.prot4824 (2007).
Luo, M.C. et al. An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Mol. Pain 1, 29 (2005).
Joiner, W.J. et al. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nat. Neurosci. 1, 462–469 (1998).
Bhattacharjee, A. et al. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J. Neurosci. 23, 11681–11691 (2003).
Budelli, G. et al. Na+-activated K+ channels express a large delayed outward current in neurons during normal physiology. Nat. Neurosci. 12, 745–750 (2009).
Renganathan, M., Cummins, T.R. & Waxman, S.G. Contribution of Nav1.8 sodium channels to action potential electrogenesis in DRG neurons. J. Neurophysiol. 86, 629–640 (2001).
Fang, X. et al. The presence and role of the tetrodotoxin-resistant sodium channel Nav1.9 (NaN) in nociceptive primary afferent neurons. J. Neurosci. 22, 7425–7433 (2002).
Fang, X., McMullan, S., Lawson, S.N. & Djouhri, L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J. Physiol. (Lond.) 565, 927–943 (2005).
Lu, B. et al. Generation of rat mutants using a coat color-tagged Sleeping Beauty transposon system. Mamm. Genome 18, 338–346 (2007).
Cavanaugh, D.J. et al. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J. Neurosci. 31, 10119–10127 (2011).
Kittler, R. et al. Genome-wide resources of endoribonuclease-prepared short interfering RNAs for specific loss-of-function studies. Nat. Methods 4, 337–344 (2007).
Brown, M.R. et al. Amino-termini isoforms of the Slack K+ channel, regulated by alternative promoters, differentially modulate rhythmic firing and adaptation. J. Physiol. (Lond.) 586, 5161–5179 (2008).
Yang, B., Desai, R. & Kaczmarek, L.K. Slack and Slick KNa channels regulate the accuracy of timing of auditory neurons. J. Neurosci. 27, 2617–2627 (2007).
Beaudoin, G.M. III et al. Afadin, a Ras/Rap effector that controls cadherin function, promotes spine and excitatory synapse density in the hippocampus. J. Neurosci. 32, 99–110 (2012).
Dani, A., Huang, B., Bergan, J., Dulac, C. & Zhuang, X. Superresolution imaging of chemical synapses in the brain. Neuron 68, 843–856 (2010).
Acknowledgements
We thank M. Tynan La Fontaine and D. Wang for the assistance with the rodent genotyping and husbandry. We thank T. Jin, H. Yang, W. Zhang, W. Ge, T. Wang, C. Peters, S. Xiao, T. Cheng, J. Berg and Z. Guan for technical help and discussions. We thank J. Crawford at Transposagen for administrative assistance. This study is supported by grants from the US National Institutes of Health (NIH) to L.Y.J. and A.I.B. and grants from the NIH and the Commonwealth of Kentucky to E.M.O. Y.-N.J. and L.Y.J. are supported by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
F.H. carried out the immunocytochemistry, molecular biology and electrophysiological studies and analyzed the data; F.H. and X.W. carried out behavioral studies and analyzed the data. T.N. and B.H. performed the STORM imaging and data analysis. E.M.O. generated the knockout rats. F.H., L.Y.J. and A.I.B. wrote the manuscript. L.Y.J. and Y.-N.J. supervised the studies.
Corresponding author
Ethics declarations
Competing interests
E.M.O. is employed by Transposagen Biopharmaceuticals, Inc.
Supplementary information
Supplementary Figures and Text
Supplementary Figures 1–9 (PDF 1073 kb)
Rights and permissions
About this article
Cite this article
Huang, F., Wang, X., Ostertag, E. et al. TMEM16C facilitates Na+-activated K+ currents in rat sensory neurons and regulates pain processing. Nat Neurosci 16, 1284–1290 (2013). https://doi.org/10.1038/nn.3468
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3468
This article is cited by
-
A Role for Transmembrane Protein 16C/Slack Impairment in Excitatory Nociceptive Synaptic Plasticity in the Pathogenesis of Remifentanil-induced Hyperalgesia in Rats
Neuroscience Bulletin (2021)
-
Rat models of human diseases and related phenotypes: a systematic inventory of the causative genes
Journal of Biomedical Science (2020)
-
Sodium-activated potassium channels shape peripheral auditory function and activity of the primary auditory neurons in mice
Scientific Reports (2019)
-
ANO9/TMEM16J promotes tumourigenesis via EGFR and is a novel therapeutic target for pancreatic cancer
British Journal of Cancer (2017)
-
A Pore Idea: the ion conduction pathway of TMEM16/ANO proteins is composed partly of lipid
Pflügers Archiv - European Journal of Physiology (2016)