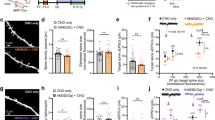
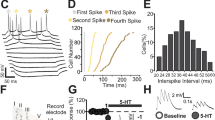
Abstract
The causes of major depression remain unknown. Antidepressants elevate concentrations of monoamines, particularly serotonin, but it remains uncertain which downstream events are critical to their therapeutic effects. We found that endogenous serotonin selectively potentiated excitatory synapses formed by the temporoammonic pathway with CA1 pyramidal cells via activation of serotonin receptors (5-HT1BRs), without affecting nearby Schaffer collateral synapses. This potentiation was expressed postsynaptically by AMPA-type glutamate receptors and required calmodulin-dependent protein kinase–mediated phosphorylation of GluA1 subunits. Because they share common expression mechanisms, long-term potentiation and serotonin-induced potentiation occluded each other. Long-term consolidation of spatial learning, a function of temporoammonic-CA1 synapses, was enhanced by 5-HT1BR antagonists. Serotonin-induced potentiation was quantitatively and qualitatively altered in a rat model of depression, restored by chronic antidepressants, and required for the ability of chronic antidepressants to reverse stress-induced anhedonia. Changes in serotonin-mediated potentiation, and its recovery by antidepressants, implicate excitatory synapses as a locus of plasticity in depression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Krishnan, V. & Nestler, E.J. The molecular neurobiology of depression. Nature 455, 894–902 (2008).
Billings, A.G., Cronkite, R.C. & Moos, R.H. Social-environmental factors in unipolar depression: comparisons of depressed patients and nondepressed controls. J. Abnorm. Psychol. 92, 119–133 (1983).
Heninger, G.R., Delgado, P.L. & Charney, D.S. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 29, 2–11 (1996).
Duman, R.S. & Aghajanian, G.K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338, 68–72 (2012).
Sanacora, G., Zarate, C.A., Krystal, J.H. & Manji, H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 7, 426–437 (2008).
Yuen, E.Y. et al. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73, 962–977 (2012).
Campbell, S. & Macqueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 29, 417–426 (2004).
Hickie, I. et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br. J. Psychiatry 186, 197–202 (2005).
Phillipson, O.T. & Griffiths, A.C. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience 16, 275–296 (1985).
Lim, B.K., Huang, K.W., Grueter, B.A., Rothwell, P.E. & Malenka, R.C. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 (2012).
Ihara, N., Ueda, S., Kawata, M. & Sano, Y. Immunohistochemical demonstration of serotonin-containing nerve fibers in the mammalian hippocampal formation. Acta Anat. 132, 335–346 (1988).
Remondes, M. & Schuman, E.M. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature 431, 699–703 (2004).
Lambe, E.K., Goldman-Rakic, P.S. & Aghajanian, G.K. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb. Cortex 10, 974–980 (2000).
Kobayashi, K., Ikeda, Y., Haneda, E. & Suzuki, H. Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J. Neurosci. 28, 6272–6280 (2008).
Aït Amara, D., Segu, L., Naili, S. & Buhot, M.C. Serotonin 1B receptor regulation after dorsal subiculum deafferentation. Brain Res. Bull. 38, 17–23 (1995).
Sari, Y. et al. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience 88, 899–915 (1999).
Saudou, F. et al. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 265, 1875–1878 (1994).
Svenningsson, P. et al. Alterations in 5-HT1B receptor function by p11 in depressive-like states. Science 311, 77–80 (2006).
Göthert, M. et al. 5-HT3 receptor antagonism by anpirtoline, a mixed 5-HT1 receptor agonist/5-HT3 receptor antagonist. Br. J. Pharmacol. 114, 269–274 (1995).
Dewar, K.M., Grondin, L., Carli, M., Lima, L. & Reader, T.A. [3H]paroxetine binding and serotonin content of rat cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment. J. Neurochem. 58, 250–257 (1992).
Sharp, T., Bramwell, S.R. & Grahame-Smith, D.G. 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br. J. Pharmacol. 96, 283–290 (1989).
Jarsky, T., Roxin, A., Kath, W.L. & Spruston, N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat. Neurosci. 8, 1667–1676 (2005).
Cai, X. et al. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44, 351–364 (2004).
Hsu, E.H., Lochan, A.C. & Cowen, D.S. Activation of Akt1 by human 5-hydroxytryptamine (serotonin)1B receptors is sensitive to inhibitors of MEK. J. Pharmacol. Exp. Ther. 298, 825–832 (2001).
Leone, A.M., Errico, M., Lin, S.L. & Cowen, D.S. Activation of extracellular signal-regulated kinase (ERK) and Akt by human serotonin 5-HT(1B) receptors in transfected BE(2)-C neuroblastoma cells is inhibited by RGS4. J. Neurochem. 75, 934–938 (2000).
Giese, K.P., Fedorov, N.B., Filipkowski, R.K. & Silva, A.J. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279, 870–873 (1998).
Roche, K.W., O'Brien, R.J., Mammen, A.L., Bernhardt, J. & Huganir, R.L. Characterization of multiple phosphorylation sites on the AMPA receptor GluA1 subunit. Neuron 16, 1179–1188 (1996).
Lee, H.K., Barbarosie, M., Kameyama, K., Bear, M.F. & Huganir, R.L. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405, 955–959 (2000).
Lee, H.-K., Takamiya, K., Hen, K., Song, L. & Huganir, R.L. Specific roles of AMPA receptor subunit GluR1(GluA1) phosphorylation sites in regulation synaptic plasticity in the CA1 region of the hippocampus. J. Neurophysiol. 103, 479–489 (2010).
Malinow, R. & Malenka, R.C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 (2002).
Sybirska, E., Davachi, L. & Goldman-Rakic, P.S. Prominence of direct entorhinal-CA1 pathway activation in sensorimotor and cognitive tasks revealed by 2-DG functional mapping in nonhuman primate. J. Neurosci. 20, 5827–5834 (2000).
Nakashiba, T., Young, J.Z., McHugh, T.J., Buhl, D.L. & Tonegawa, S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319, 1260–1264 (2008).
Tatarczyńska, E., Kłodzińska, A., Stachowicz, K. & Chojnacka-Wójcik, E. Effects of a selective 5–HT1B receptor agonist and antagonists in animal models of anxiety and depression. Behav. Pharmacol. 15, 523–534 (2004).
Buhot, M.C. et al. Spatial learning in the 5–HT1B receptor knockout mouse: selective facilitation/impairment depending on the cognitive demand. Learn. Mem. 10, 466–477 (2003).
Willner, P., Towell, A., Sampson, D., Sophokleous, S. & Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl.) 93, 358–364 (1987).
David, D.J. et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62, 479–493 (2009).
Svenningsson, P. et al. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac). Proc. Natl. Acad. Sci. USA 99, 3182–3187 (2002).
Bondi, C.O., Rodriguez, G., Gould, G.G., Frazer, A. & Morilak, D.A. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33, 320–331 (2008).
Malatynska, E. & Knapp, R. Dominant-submissive behavior as models of mania and depression. Neurosci. Biobehav. Rev. 29, 715–737 (2005).
Gaster, L.M. et al. The selective 5–HT1B receptor inverse agonist 1′-methyl-5-[[2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydro-spiro[furo[2,3-f]indole-3,4′-piperidine](SB-224289) potently blocks terminal 5-HT autoreceptor function both in vitro and in vivo. J. Med. Chem. 41, 1218–1235 (1998).
Bechtholt, A.J., Smith, K., Gaughan, S. & Lucki, I. Sucrose intake and fasting glucose levels in 5-HT(1A) and 5-HT(1B) receptor mutant mice. Physiol. Behav. 93, 659–665 (2008).
O'Neill, M.F. & Conway, M.W. Role of 5-HT1A and 5-HT1B receptors in the mediation of behavior in the forced swim test in mice. Neuropsychopharmacology 24, 391–398 (2001).
Buard, I. et al. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J. Neurosci. 30, 8214–8220 (2010).
Krugers, H.J., Lucassen, P.J., Karst, H. & Joëls, M. Chronic stress effects on hippocampal structure and synaptic function: relevance for depression and normalization by anti-glucocorticoid treatment. Front. Synaptic Neurosci. 2, 1–10 (2010).
Pavlides, C., Nivon, L.G. & McEwen, B.S. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus 12, 245–257 (2002).
Boulenguez, P. et al. Modulation of dopamine release in the nucleus accumbens by 5HTlB agonists: Involvement of the hippocampo-accumbens pathway. Neuropharmacology 35, 1521–1529 (1996).
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013).
Chourbaji, S. et al. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J. 22, 3129–3134 (2008).
Autry, A.E. et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95 (2011).
Santarelli, L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 (2003).
Acknowledgements
We thank S. Ahmari and R. Hen (Columbia University) for providing Hrt1b−/− mice, B. Alger, T. Blanpied, T. Gould, J. Kim and M. McCarthy for their comments, S. Aungst for advice on immunohistochemistry, and H. Zimmerman, L. Mok and M. Taylor for technical assistance. This work was funded by a Mr. and Mrs. Robert and Lee Peterson Southwest Florida National Alliance for Research on Schizophrenia and Depression Young Investigator Award (X.C.), the Howard Hughes Medical Institute (R.L.H.) and the US National Institutes of Health (R.L.H., H.-K.L., X.C. and S.M.T.).
Author information
Authors and Affiliations
Contributions
X.C., A.J.K., M.D.K., A.M.B. and S.M.T. designed the study. X.C., A.J.K., M.D.K., S.G., K.G. and A.M.B. performed the experiments and analyzed the data. H.-K.L. and R.L.H. provided the GluA1 S831A mice. X.C., A.J.K. and S.M.T. prepared the manuscript. All of the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 8719 kb)
Rights and permissions
About this article
Cite this article
Cai, X., Kallarackal, A., Kvarta, M. et al. Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression. Nat Neurosci 16, 464–472 (2013). https://doi.org/10.1038/nn.3355
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3355
This article is cited by
-
Single-nucleus transcriptomic analysis reveals the relationship between gene expression in oligodendrocyte lineage and major depressive disorder
Journal of Translational Medicine (2024)
-
16S rRNA gene sequencing reveals the effect of fluoxetine on gut microbiota in chronic unpredictable stress-induced depressive-like rats
Annals of General Psychiatry (2023)
-
SSRIs differentially modulate the effects of pro-inflammatory stimulation on hippocampal plasticity and memory via sigma 1 receptors and neurosteroids
Translational Psychiatry (2023)
-
Plasticity of synapses and reward circuit function in the genesis and treatment of depression
Neuropsychopharmacology (2023)
-
Receptor-informed network control theory links LSD and psilocybin to a flattening of the brain’s control energy landscape
Nature Communications (2022)