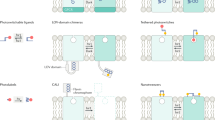
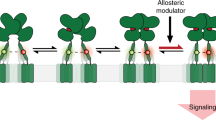
Abstract
G protein–coupled receptors (GPCRs), the largest family of membrane signaling proteins, respond to neurotransmitters, hormones and small environmental molecules. The neuronal function of many GPCRs has been difficult to resolve because of an inability to gate them with subtype specificity, spatial precision, speed and reversibility. To address this, we developed an approach for opto-chemical engineering of native GPCRs. We applied this to the metabotropic glutamate receptors (mGluRs) to generate light-agonized and light-antagonized mGluRs (LimGluRs). The light-agonized LimGluR2, on which we focused, was fast, bistable and supported multiple rounds of on/off switching. Light gated two of the primary neuronal functions of mGluR2: suppression of excitability and inhibition of neurotransmitter release. We found that the light-antagonized tool LimGluR2-block was able to manipulate negative feedback of synaptically released glutamate on transmitter release. We generalized the optical control to two additional family members: mGluR3 and mGluR6. This system worked in rodent brain slices and in zebrafish in vivo, where we found that mGluR2 modulated the threshold for escape behavior. These light-gated mGluRs pave the way for determining the roles of mGluRs in synaptic plasticity, memory and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Szobota, S. & Isacoff, E.Y. Optical control of neuronal activity. Annu. Rev. Biophys. 39, 329–348 (2010).
Deisseroth, K. Optogenetics. Nat. Methods 8, 26–29 (2011).
Pierce, K.L., Premont, R.T. & Lefkowitz, R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 (2002).
Kobilka, B.K. Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol. Sci. 32, 213–218 (2011).
Niswender, C.M. & Conn, P.J. Metabotropic glutamate receptors: physiology, pharmacology and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322 (2010).
Bockaert, J., Perroy, J., Becamel, C., Marin, P. & Fagni, L. GPCR interacting proteins (GIPs) in the nervous system: roles in physiology and pathologies. Annu. Rev. Pharmacol. Toxicol. 50, 89–109 (2010).
Gainetdinov, R.R., Premont, R.T., Bohn, L.M., Lefkowitz, R.J. & Caron, M.G. Desensitization of G protein–coupled receptors and neuronal functions. Annu. Rev. Neurosci. 27, 107–144 (2004).
Pei, Y., Rogan, S.C., Yan, F. & Roth, B.L. Engineered GPCRs as tools to modulate signal transduction. Physiology (Bethesda) 23, 313–321 (2008).
Alexander, G.M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein–coupled receptors. Neuron 63, 27–39 (2009).
Zemelman, B.V., Lee, G.A., Ng, M. & Miesenbock, G. Selective photostimulation of genetically chARGed neurons. Neuron 33, 15–22 (2002).
Li, X. et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA 102, 17816–17821 (2005).
Gutierrez, D.V. et al. Optogenetic control of motor coordination by Gi/o protein–coupled vertebrate rhodopsin in cerebellar Purkinje cells. J. Biol. Chem. 286, 25848–25858 (2011).
Melyan, Z., Tarttelin, E.E., Bellingham, J., Lucas, R.J. & Hankins, M.W. Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433, 741–745 (2005).
Qiu, X. et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature 433, 745–749 (2005).
Lin, B., Koizumi, A., Tanaka, N., Panda, S. & Masland, R.H. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc. Natl. Acad. Sci. USA 105, 16009–16014 (2008).
Yamashita, T., Terakita, A. & Shichida, Y. Distinct roles of the second and third cytoplasmic loops of bovine rhodopsin in G protein activation. J. Biol. Chem. 275, 34272–34279 (2000).
Yamashita, T., Terakita, A. & Shichida, Y. The second cytoplasmic loop of metabotropic glutamate receptor functions at the third loop position of rhodopsin. J. Biochem. 130, 149–155 (2001).
Kim, J.M. et al. Light-driven activation of beta 2-adrenergic receptor signaling by a chimeric rhodopsin containing the beta 2-adrenergic receptor cytoplasmic loops. Biochemistry 44, 2284–2292 (2005).
Airan, R.D., Thompson, K.R., Fenno, L.E., Bernstein, H. & Deisseroth, K. Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 (2009).
Oh, E., Maejima, T., Liu, C., Deneris, E. & Herlitze, S. Substitution of 5-HT1A receptor signaling by a light-activated G protein–coupled receptor. J. Biol. Chem. 285, 30825–30836 (2010).
Bailes, H.J., Zhuang, L.Y. & Lucas, R.J. Reproducible and sustained regulation of Galphas signaling using a metazoan opsin as an optogenetic tool. PLoS ONE 7, e30774 (2012).
Fehrentz, T., Schonberger, M. & Trauner, D. Optochemical genetics. Angew. Chem. Int. Edn Engl. 50, 12156–12182 (2011).
Banghart, M., Borges, K., Isacoff, E., Trauner, D. & Kramer, R.H. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7, 1381–1386 (2004).
Volgraf, M. et al. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol. 2, 47–52 (2006).
Anwyl, R. Metabotropic glutamate receptor–dependent long-term potentiation. Neuropharmacology 56, 735–740 (2009).
Lüscher, C. & Huber, K.M. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65, 445–459 (2010).
Panatier, A. et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798 (2011).
Bradley, S.J. & Challiss, R.A. G protein–coupled receptor signaling in astrocytes in health and disease: a focus on metabotropic glutamate receptors. Biochem. Pharmacol. 84, 249–259 (2012).
Tanabe, Y., Masu, M., Ishii, T., Shigemoto, R. & Nakanishi, S. A family of metabotropic glutamate receptors. Neuron 8, 169–179 (1992).
Saugstad, J.A., Segerson, T.P. & Westbrook, G.L. Metabotropic glutamate receptors activate G protein–coupled inwardly rectifying potassium channels in Xenopus oocytes. J. Neurosci. 16, 5979–5985 (1996).
Ikeda, S.R., Lovinger, D.M., McCool, B.A. & Lewis, D.L. Heterologous expression of metabotropic glutamate receptors in adult rat sympathetic neurons: subtype-specific coupling to ion channels. Neuron 14, 1029–1038 (1995).
Altinbilek, B. & Manahan-Vaughan, D. A specific role for group II metabotropic glutamate receptors in hippocampal long-term depression and spatial memory. Neuroscience 158, 149–158 (2009).
Marek, G.J. Metabotropic glutamate 2/3 receptors as drug targets. Curr. Opin. Pharmacol. 4, 18–22 (2004).
Muto, T., Tsuchiya, D., Morikawa, K. & Jingami, H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 104, 3759–3764 (2007).
Tsuji, T., Takashima, H., Takeuchi, H., Egawa, T. & Konaka, S. Molecular structure and torsional potential of trans-azobenzene. A gas electron diffraction study. J. Phys. Chem. A 105, 9347–9353 (2001).
Chang, G., Guida, W.C. & Still, W.C. An internal coordinate Monte-Carlo method for searching conformational space. J. Am. Chem. Soc. 111, 4379–4386 (1989).
Gorostiza, P. et al. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc. Natl. Acad. Sci. USA 104, 10865–10870 (2007).
Janovjak, H., Szobota, S., Wyart, C., Trauner, D. & Isacoff, E.Y. A light-gated, potassium-selective glutamate receptor for the optical inhibition of neuronal firing. Nat. Neurosci. 13, 1027–1032 (2010).
Iacovelli, L. et al. Regulation of group II metabotropic glutamate receptors by G protein–coupled receptor kinases: mGlu2 receptors are resistant to homologous desensitization. Mol. Pharmacol. 75, 991–1003 (2009).
Raveh, A., Cooper, A., Guy-David, L. & Reuveny, E. Nonenzymatic rapid control of GIRK channel function by a G protein–coupled receptor kinase. Cell 143, 750–760 (2010).
Hanna, L. et al. Differentiating the roles of mGlu2 and mGlu3 receptors using LY541850, an mGlu2 agonist/mGlu3 antagonist. Neuropharmacology 66, 114–121 (2013).
Ehrengruber, M.U. et al. Activation of heteromeric G protein–gated inward rectifier K+ channels overexpressed by adenovirus gene transfer inhibits the excitability of hippocampal neurons. Proc. Natl. Acad. Sci. USA 94, 7070–7075 (1997).
Takahashi, T., Forsythe, I.D., Tsujimoto, T., Barnes-Davies, M. & Onodera, K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science 274, 594–597 (1996).
Shigemoto, R. et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 17, 7503–7522 (1997).
Leaney, J.L. Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein–gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur. J. Neurosci. 18, 2110–2118 (2003).
Koch, M. The neurobiology of startle. Prog. Neurobiol. 59, 107–128 (1999).
Burgess, H.A. & Granato, M. Sensorimotor gating in larval zebrafish. J. Neurosci. 27, 4984–4994 (2007).
Caporale, N. et al. LiGluR restores visual responses in rodent models of inherited blindness. Mol. Ther. 19, 1212–1219 (2011).
Grauer, S.M. & Marquis, K.L. Intracerebral administration of metabotropic glutamate receptor agonists disrupts prepulse inhibition of acoustic startle in Sprague-Dawley rats. Psychopharmacology (Berl.) 141, 405–412 (1999).
Haug, M.F., Gesemann, M., Mueller, T. & Neuhauss, S.C. Phylogeny and expression divergence of metabotropic glutamate receptor genes in the brain of zebrafish (Danio rerio). J. Comp. Neurol. Published online, doi:10.1002/cne.23240 (10 October 2012).
Bordoli, L. et al. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 4, 1–13 (2009).
Kaminski, G.A., Friesner, R.A., Tirado-Rives, J. & Jorgensen, W.L. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B 105, 6474–6487 (2001).
Davis, I.W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, 375–383 (2007).
Vivaudou, M. et al. Probing the G-protein regulation of GIRK1 and GIRK4, the two subunits of the KACh channel, using functional homomeric mutants. J. Biol. Chem. 272, 31553–31560 (1997).
Bekkers, J.M. & Stevens, C.F. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc. Natl. Acad. Sci. USA 88, 7834–7838 (1991).
Kwan, K.M. et al. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 (2007).
Kurita, R. et al. Suppression of lens growth by alphaA-crystallin promoter–driven expression of diphtheria toxin results in disruption of retinal cell organization in zebrafish. Dev. Biol. 255, 113–127 (2003).
Grabher, C. & Wittbrodt, J. Meganuclease and transposon mediated transgenesis in medaka. Genome Biol. 8 (suppl. 1), S10 (2007).
Acknowledgements
We thank A.P. Mariani (National Eye Institute) for 11-cis retinal, J.P. Pin (University of Montpellier) for the mGluR plasmids and E. Reuveny (Weizmann Institute) for the GIRK1 plasmid, K. Durkin, K. Dubay, T. Berger, G. Sandoz and S. Berlin for helpful discussions, A. Guyon, Z. Fu and S. Szobota for help with slice cultures, Z. Fu for molecular biology assistance, K. McDaniel, J. Maxfield, J. Saint-Hillaire and D. Weinman for fish care, E. Carroll for discussion and help with zebrafish set up, P. Gut (University of California, San Francisco) for zebrafish plasmids, H. Baier (University of California, San Francisco) for fish lines, and the College of Chemistry (University of California, Berkeley) for computing resources for the Monte Carlo simulations. Support for the work was provided by the Nanomedicine Development Center for the Optical Control of Biological Function, US National Institutes of Health grant PN2EY018241 (D.T. and E.Y.I.), the Human Frontier Science Program (RGP0013/2010 to E.Y.I.), the Deutsche Forschungsgemeinschaft (FOR 1279, D.T.), the Fond der Chemischen Industrie (Kekulé fellowship to P.S.), National Science Foundation grants CHE-0233882 and CHE-0840505 (to the College of Chemistry at the University of California, Berkeley), a postdoctoral fellowship of the European Molecular Biology Organization (H.J.), a Helen Hay Whitney postdoctoral fellowship (D.S.), the McKnight Endowment Fund for Neuroscience and US National Institutes of Health grant R01HL109525 (A.F.S.), and a predoctoral fellowship from the Fulbright Foundation (B.G.).
Author information
Authors and Affiliations
Contributions
J.L. designed experiments and conducted homology modeling, Monte Carlo simulations, HEK293 cell patch experiments, cAMP measurements, cultured neuron and hippocampal slice patch experiments, analyzed data, and wrote the manuscript. C.P. designed zebrafish experiments, conducted zebrafish behavioral experiments, analyzed data, and wrote the manuscript. B.G. and A.R. conducted HEK293 cell patch experiments. H.J. designed and conducted Monte Carlo simulations. P.S. and B.K. synthesized photoswitches. A.H., D.S. and A.F.S. developed and setup zebrafish behavioral apparatus. D.T. developed photoswitching methodology and provided photoswitches. E.Y.I. supervised the project, designed experiments, analyzed data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10, Supplementary Table 1 and Chemical Synthesis (PDF 1954 kb)
Rights and permissions
About this article
Cite this article
Levitz, J., Pantoja, C., Gaub, B. et al. Optical control of metabotropic glutamate receptors. Nat Neurosci 16, 507–516 (2013). https://doi.org/10.1038/nn.3346
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3346
This article is cited by
-
Optical control of neuronal ion channels and receptors
Nature Reviews Neuroscience (2019)
-
Conformational pathway provides unique sensitivity to a synaptic mGluR
Nature Communications (2019)
-
Restoration of high-sensitivity and adapting vision with a cone opsin
Nature Communications (2019)
-
Holographic two-photon activation for synthetic optogenetics
Nature Protocols (2019)