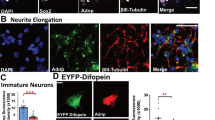
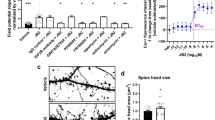
Abstract
L-type voltage gated calcium channels have an important role in neuronal development by promoting dendritic growth and arborization. A point mutation in the gene encoding CaV1.2 causes Timothy syndrome, a neurodevelopmental disorder associated with autism spectrum disorders (ASDs). We report that channels with the Timothy syndrome alteration cause activity-dependent dendrite retraction in rat and mouse neurons and in induced pluripotent stem cell (iPSC)-derived neurons from individuals with Timothy syndrome. Dendrite retraction was independent of calcium permeation through the mutant channel, was associated with ectopic activation of RhoA and was inhibited by overexpression of the channel-associated GTPase Gem. These results suggest that CaV1.2 can activate RhoA signaling independently of Ca2+ and provide insights into the cellular basis of Timothy syndrome and other ASDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Dolmetsch, R. Excitation-transcription coupling: signaling by ion channels to the nucleus. Sci. STKE 2003, PE4 (2003).
Tonelli, A. et al. Early onset, non fluctuating spinocerebellar ataxia and a novel missense mutation in CACNA1A gene. J. Neurol. Sci. 241, 13–17 (2006).
Wang, K. et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 459, 528–533 (2009).
Nyegaard, M. et al. CACNA1C (rs1006737) is associated with schizophrenia. Mol. Psychiatry 15, 119–121 (2010).
Tottene, A. et al. Familial hemiplegic migraine mutations increase Ca(2+) influx through single human CaV2.1 channels and decrease maximal CaV2.1 current density in neurons. Proc. Natl. Acad. Sci. USA 99, 13284–13289 (2002).
Splawski, I. et al. CACNA1H mutations in autism spectrum disorders. J. Biol. Chem. 281, 22085–22091 (2006).
Barrett, C.F. & Tsien, R.W. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc. Natl. Acad. Sci. USA 105, 2157–2162 (2008).
Hoda, J.C., Zaghetto, F., Singh, A., Koschak, A. & Striessnig, J. Effects of congenital stationary night blindness type 2 mutations R508Q and L1364H on Cav1.4 L-type Ca2+ channel function and expression. J. Neurochem. 96, 1648–1658 (2006).
Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 (2000).
Richards, M.W., Butcher, A.J. & Dolphin, A.C. Ca2+ channel beta-subunits: structural insights AID our understanding. Trends Pharmacol. Sci. 25, 626–632 (2004).
Pravettoni, E. et al. Different localizations and functions of L-type and N-type calcium channels during development of hippocampal neurons. Dev. Biol. 227, 581–594 (2000).
Hell, J.W. et al. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J. Cell Biol. 123, 949–962 (1993).
Splawski, I. et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119, 19–31 (2004).
McAllister, A.K., Katz, L.C. & Lo, D.C. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 17, 1057–1064 (1996).
Redmond, L., Kashani, A.H. & Ghosh, A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron 34, 999–1010 (2002).
Wong, R.O. & Ghosh, A. Activity-dependent regulation of dendritic growth and patterning. Nat. Rev. Neurosci. 3, 803–812 (2002).
Aizawa, H. et al. Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science 303, 197–202 (2004).
Lohmann, C. & Wong, R.O. Regulation of dendritic growth and plasticity by local and global calcium dynamics. Cell Calcium 37, 403–409 (2005).
Fink, C.C. et al. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron 39, 283–297 (2003).
Lohmann, C., Myhr, K.L. & Wong, R.O. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature 418, 177–181 (2002).
Oyama, F. et al. Gem GTPase and tau: morphological changes induced by gem GTPase in cho cells are antagonized by tau. J. Biol. Chem. 279, 27272–27277 (2004).
Mahalakshmi, R.N. et al. Nuclear localization of endogenous RGK proteins and modulation of cell shape remodeling by regulated nuclear transport. Traffic 8, 1164–1178 (2007).
Finlin, B.S. et al. Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J. Biol. Chem. 280, 41864–41871 (2005).
Beguin, P. et al. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature 411, 701–706 (2001).
Beguin, P. et al. RGK small GTP-binding proteins interact with the nucleotide kinase domain of Ca2+-channel beta-subunits via an uncommon effector binding domain. J. Biol. Chem. 282, 11509–11520 (2007).
Beguin, P. et al. 14–3-3 and calmodulin control subcellular distribution of Kir/Gem and its regulation of cell shape and calcium channel activity. J. Cell Sci. 118, 1923–1934 (2005).
Finlin, B.S. et al. Analysis of the complex between Ca2+ channel beta-subunit and the Rem GTPase. J. Biol. Chem. 281, 23557–23566 (2006).
Aresta, S., de Tand-Heim, M.F., Beranger, F. & de Gunzburg, J. A novel Rho GTPase-activating-protein interacts with Gem, a member of the Ras superfamily of GTPases. Biochem. J. 367, 57–65 (2002).
Ward, Y. et al. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J. Cell Biol. 157, 291–302 (2002).
Bader, P.L. et al. Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proc. Natl. Acad. Sci. USA 108, 15432–15437 (2011).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Dolmetsch, R. & Geschwind, D.H. The human brain in a dish: the promise of iPSC-derived neurons. Cell 145, 831–834 (2011).
Yazawa, M. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011).
Pasca, S.P. et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med. 17, 1657–1662 (2011).
Splawski, I. et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl. Acad. Sci. USA 102, 8089–8096 (2005).
Dolmetsch, R.E., Pajvani, U., Fife, K., Spotts, J.M. & Greenberg, M.E. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 294, 333–339 (2001).
Yang, J., Ellinor, P.T., Sather, W.A., Zhang, J.F. & Tsien, R.W. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature 366, 158–161 (1993).
Parent, L. & Gopalakrishnan, M. Glutamate substitution in repeat IV alters divalent and monovalent cation permeation in the heart Ca2+ channel. Biophys. J. 69, 1801–1813 (1995).
Lee, T., Winter, C., Marticke, S.S., Lee, A. & Luo, L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron 25, 307–316 (2000).
Swetman, C.A. et al. Extension, retraction and contraction in the formation of a dendritic cell dendrite: distinct roles for Rho GTPases. Eur. J. Immunol. 32, 2074–2083 (2002).
Kranenburg, O. et al. Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: induction of neurite retraction. Mol. Biol. Cell 10, 1851–1857 (1999).
Totsukawa, G. et al. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 150, 797–806 (2000).
Casanova, M.F. et al. Minicolumnar abnormalities in autism. Acta Neuropathol. 112, 287–303 (2006).
Belmonte, M.K. et al. Autism and abnormal development of brain connectivity. J. Neurosci. 24, 9228–9231 (2004).
Geschwind, D.H. & Levitt, P. Autism spectrum disorders: developmental disconnection syndromes. Curr. Opin. Neurobiol. 17, 103–111 (2007).
Belmonte, M.K. & Bourgeron, T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat. Neurosci. 9, 1221–1225 (2006).
Kaufmann, W.E. & Moser, H.W. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex 10, 981–991 (2000).
Cohen-Kutner, M., Nachmanni, D. & Atlas, D. CaV2.1 (P/Q channel) interaction with synaptic proteins is essential for depolarization-evoked release. Channels (Austin) 4, 266–277 (2010).
Wiser, O. et al. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc. Natl. Acad. Sci. USA 96, 248–253 (1999).
Tanabe, T., Beam, K.G., Adams, B.A., Niidome, T. & Numa, S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature 346, 567–569 (1990).
Green, E.M., Barrett, C.F., Bultynck, G., Shamah, S.M. & Dolmetsch, R.E. The tumor suppressor eIF3e mediates calcium-dependent internalization of the L-type calcium channel CaV1.2. Neuron 55, 615–632 (2007).
Gomez-Ospina, N., Tsuruta, F., Barreto-Chang, O., Hu, L. & Dolmetsch, R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell 127, 591–606 (2006).
Yuan, B., Latek, R., Hossbach, M., Tuschl, T. & Lewitter, F. siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucl. Acids Res. 32, W130–W134 (2004).
Paradis, S. et al. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron 53, 217–232 (2007).
Xia, Z., Dudek, H., Miranti, C.K. & Greenberg, M.E. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci. 16, 5425–5436 (1996).
Kaech, S. & Banker, G. Culturing hippocampal neurons. Nat. Protoc. 1, 2406–2415 (2006).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007).
Meijering, E. et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58, 167–176 (2004).
Acknowledgements
We thank K. Timothy and the individuals with Timothy syndrome who participated in this study, J. Bernstein and J. Hallmayer for recruiting the subjects for this study, and E. Nigh for critical reading of the manuscript. J.F.K. was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award (F31 NS055549-03) from the National Institute of Neurological Disorders and Stroke. Financial support was provided by a US National Institutes of Health Director's Pioneer Award and a Simons Foundation Grant to R.E.D.; the International Brain Research Organization Outstanding Research Fellowship and the Tashia and John Morgridge Endowed Fellowship to S.P.P.; a Japan Society of the Promotion for Science Postdoctoral Fellowship for Research Abroad and American Heart Association Western States to M.Y.; and a California Institute for Regenerative Medicine Postdoctoral Fellowship to O.S. We are grateful for funding from B. and F. Horowitz, M. McCafferey, B. and J. Packard, P. Kwan and K. Wang.
Author information
Authors and Affiliations
Contributions
R.E.D. and J.F.K. designed the experiments and wrote the manuscript; J.F.K. performed all of the cellular assays in rodent cells, including the calcium imaging and immunocytochemistry studies, all of the mice breeding and the in vivo studies of dendritic arborization; S.P.P. differentiated iPSCs into neurons and performed the dendritic arborization experiments in human cells; A.S. performed and analyzed the electrophysiology experiments; M.Y. generated and characterized the iPSCs; R.S. contributed to the analysis of dendrites in iPSC-derived neurons; R.R. generated the Timothy syndrome mice.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 17281 kb)
Rights and permissions
About this article
Cite this article
Krey, J., Paşca, S., Shcheglovitov, A. et al. Timothy syndrome is associated with activity-dependent dendritic retraction in rodent and human neurons. Nat Neurosci 16, 201–209 (2013). https://doi.org/10.1038/nn.3307
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3307
This article is cited by
-
Modeling human neurodevelopmental diseases with brain organoids
Cell Regeneration (2022)
-
iPSC toolbox for understanding and repairing disrupted brain circuits in autism
Molecular Psychiatry (2022)
-
Maturation and circuit integration of transplanted human cortical organoids
Nature (2022)
-
Cytosolic peptides encoding CaV1 C-termini downregulate the calcium channel activity-neuritogenesis coupling
Communications Biology (2022)
-
Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications
Signal Transduction and Targeted Therapy (2022)