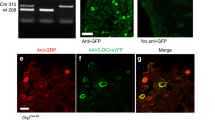
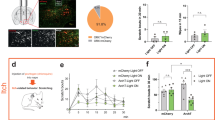
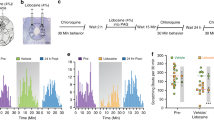
Abstract
Itch-specific neurons have been sought for decades. The existence of such neurons has been doubted recently as a result of the observation that itch-mediating neurons also respond to painful stimuli. We genetically labeled and manipulated MrgprA3+ neurons in the dorsal root ganglion (DRG) and found that they exclusively innervated the epidermis of the skin and responded to multiple pruritogens. Ablation of MrgprA3+ neurons led to substantial reductions in scratching evoked by multiple pruritogens and occurring spontaneously under chronic itch conditions, whereas pain sensitivity remained intact. Notably, mice in which TRPV1 was exclusively expressed in MrgprA3+ neurons exhibited itch, but not pain, behavior in response to capsaicin. Although MrgprA3+ neurons were sensitive to noxious heat, activation of TRPV1 in these neurons by noxious heat did not alter pain behavior. These data suggest that MrgprA3 defines a specific subpopulation of DRG neurons mediating itch. Our study opens new avenues for studying itch and developing anti-pruritic therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ikoma, A., Steinhoff, M., Stander, S., Yosipovitch, G. & Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 7, 535–547 (2006).
Basbaum, A.I., Bautista, D.M., Scherrer, G. & Julius, D. Cellular and molecular mechanisms of pain. Cell 139, 267–284 (2009).
Schmelz, M., Schmidt, R., Bickel, A., Handwerker, H.O. & Torebjork, H.E. Specific C-receptors for itch in human skin. J. Neurosci. 17, 8003–8008 (1997).
Schmelz, M. et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J. Neurophysiol. 89, 2441–2448 (2003).
Johanek, L.M. et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J. Neurosci. 28, 7659–7669 (2008).
Namer, B. et al. Separate peripheral pathways for pruritus in man. J. Neurophysiol. 100, 2062–2069 (2008).
Akiyama, T., Carstens, M.I. & Carstens, E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J. Neurophysiol. 104, 2442–2450 (2010).
Ma, C., Nie, H., Gu, Q., Sikand, P. & LaMotte, R.H. In vivo responses of cutaneous C-mechanosensitive neurons in mouse to punctate chemical stimuli that elicit itch and nociceptive sensations in humans. J. Neurophysiol. 107, 357–363 (2012).
LaMotte, R.H., Shimada, S.G., Green, B.G. & Zelterman, D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J. Neurophysiol. 101, 1430–1443 (2009).
Wilson, S.R. et al. TRPA1 is required for histamine-independent, Mas-related G protein–coupled receptor–mediated itch. Nat. Neurosci. 14, 595–602 (2011).
Liu, Q. et al. Sensory neuron–specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 1353–1365 (2009).
Sikand, P., Dong, X. & LaMotte, R.H. BAM8–22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J. Neurosci. 31, 7563–7567 (2011).
Liu, Q. et al. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci. Signal. 4, ra45 (2011).
Zylka, M.J., Dong, X., Southwell, A.L. & Anderson, D.J. Atypical expansion in mice of the sensory neuron–specific Mrg G protein–coupled receptor family. Proc. Natl. Acad. Sci. USA 100, 10043–10048 (2003).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Dong, X., Han, S., Zylka, M.J., Simon, M.I. & Anderson, D.J. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106, 619–632 (2001).
Zylka, M.J., Rice, F.L. & Anderson, D.J. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 45, 17–25 (2005).
Liu, Y. et al. Mechanisms of compartmentalized expression of Mrg class G protein–coupled sensory receptors. J. Neurosci. 28, 125–132 (2008).
Sun, Y.G. & Chen, Z.F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 (2007).
Sun, Y.G. et al. Cellular basis of itch sensation. Science 325, 1531–1534 (2009).
Barth, A.L., Gerkin, R.C. & Dean, K.L. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J. Neurosci. 24, 6466–6475 (2004).
Ma, C., Donnelly, D.F. & LaMotte, R.H. In vivo visualization and functional characterization of primary somatic neurons. J. Neurosci. Methods 191, 60–65 (2010).
Shinohara, T. et al. Identification of a G protein–coupled receptor specifically responsive to beta-alanine. J. Biol. Chem. 279, 23559–23564 (2004).
Abrahamsen, B. et al. The cell and molecular basis of mechanical, cold and inflammatory pain. Science 321, 702–705 (2008).
Naglich, J.G., Metherall, J.E., Russell, D.W. & Eidels, L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell 69, 1051–1061 (1992).
Saito, M. et al. Diphtheria toxin receptor–mediated conditional and targeted cell ablation in transgenic mice. Nat. Biotechnol. 19, 746–750 (2001).
Buch, T. et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426 (2005).
Imamachi, N. et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA 106, 11330–11335 (2009).
Di Nardo, A., Wertz, P., Giannetti, A. & Seidenari, S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm. Venereol. 78, 27–30 (1998).
Krajnik, M. & Zylicz, Z. Understanding pruritus in systemic disease. J. Pain Symptom Manage. 21, 151–168 (2001).
Miyamoto, T., Nojima, H., Shinkado, T., Nakahashi, T. & Kuraishi, Y. Itch-associated response induced by experimental dry skin in mice. Jpn. J. Pharmacol. 88, 285–292 (2002).
Saint-Mezard, P. et al. Allergic contact dermatitis. Eur. J. Dermatol. 14, 284–295 (2004).
Skoner, D.P. Allergic rhinitis: definition, epidemiology, pathophysiology, detection and diagnosis. J. Allergy Clin. Immunol. 108, S2–S8 (2001).
Ono, S.J. & Abelson, M.B. Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J. Allergy Clin. Immunol. 115, 118–122 (2005).
Shimada, S.G. & LaMotte, R.H. Behavioral differentiation between itch and pain in mouse. Pain 139, 681–687 (2008).
Liu, Q. et al. Mechanisms of itch evoked by beta-alanine. J. Neurosci. 32, 14532–14537 (2012).
Arenkiel, B.R., Klein, M.E., Davison, I.G., Katz, L.C. & Ehlers, M.D. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nat. Methods 5, 299–302 (2008).
Güler, A.D. et al. Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat. Commun. 3, 746 (2012).
Caterina, M.J. et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 (2000).
Sikand, P., Shimada, S.G., Green, B.G. & LaMotte, R.H. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain 152, 2485–2494 (2011).
Sikand, P., Shimada, S.G., Green, B.G. & LaMotte, R.H. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 144, 66–75 (2009).
Green, B.G. Spatial summation of chemical irritation and itch produced by topical application of capsaicin. Percept. Psychophys. 48, 12–18 (1990).
McQueen, D.S., Noble, M.A. & Bond, S.M. Endothelin-1 activates ETA receptors to cause reflex scratching in BALB/c mice. Br. J. Pharmacol. 151, 278–284 (2007).
Yamaguchi, T., Nagasawa, T., Satoh, M. & Kuraishi, Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci. Res. 35, 77–83 (1999).
Finger, S. & Wade, N.J. The neuroscience of Helmholtz and the theories of Johannes Muller. Part 2. Sensation and perception. J. Hist. Neurosci. 11, 234–254 (2002).
McMahon, S.B. & Koltzenburg, M. Itching for an explanation. Trends Neurosci. 15, 497–501 (1992).
Lagerström, M.C. et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron 68, 529–542 (2010).
Ross, S.E. et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65, 886–898 (2010).
Liu, Y. et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 68, 543–556 (2010).
Davidson, S. & Giesler, G.J. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 33, 550–558 (2010).
Acknowledgements
We thank C. Hawkins and the staff of Transgenic Mouse Core at Johns Hopkins University School of Medicine for assistance with BAC transgenic mouse generation. We thank D. Anderson (California Institute of Technology) and M. Zylka (University of North Carolina at Chapel Hill) for providing MrgprdGFP/+ mice and A. Guler (University of Washington) for providing Rosa26Trpv1 mice. The work was supported by grants from the US National Institutes of Health to X.D. (NS054791 and GM087369), R.L. (NS047399 and NS014624) and Y.G. (NS070814). X.D. is an Early Career Scientist of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
L.H. generated the Mrgpra3GFP-Cre mice, carried out the genetic manipulation and most of the behavioral, immunostaining and Ca2+ imaging experiments, and wrote the manuscript. C.M., H.N. and L.Q. conducted in vivo DRG recordings. Q.L., H.-J.W. and K.N.P. contributed to behavioral experiments. Y.C. and B.X. made the MrgprA3-Cre BAC construct. Z.T., Y.K. and Z.L. conducted in vitro DRG recordings. B.M., S.H. and Y.G. contributed to immunostaining experiments. R.L. and X.D. supervised the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Table 1 (PDF 1096 kb)
Rights and permissions
About this article
Cite this article
Han, L., Ma, C., Liu, Q. et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16, 174–182 (2013). https://doi.org/10.1038/nn.3289
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3289
This article is cited by
-
Cutaneous Components Leading to Pruritus, Pain, and Neurosensitivity in Atopic Dermatitis: A Narrative Review
Dermatology and Therapy (2024)
-
Peripheral signaling pathways contributing to non-histaminergic itch in humans
Journal of Translational Medicine (2023)
-
Calretinin-expressing islet cells are a source of pre- and post-synaptic inhibition of non-peptidergic nociceptor input to the mouse spinal cord
Scientific Reports (2023)
-
Intensity-adjustable pain management with prolonged duration based on phase-transitional nanoparticles-assisted ultrasound imaging-guided nerve blockade
Journal of Nanobiotechnology (2022)
-
Neuronal FcεRIα directly mediates ocular itch via IgE-immune complex in a mouse model of allergic conjunctivitis
Journal of Neuroinflammation (2022)