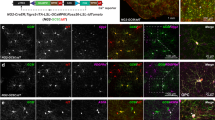
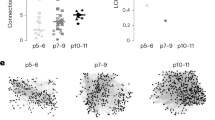
Abstract
Developing axons must control their growth rate to follow the appropriate pathways and establish specific connections. However, the regulatory mechanisms involved remain elusive. By combining live imaging with transplantation studies in mice, we found that spontaneous calcium activity in the thalamocortical system and the growth rate of thalamocortical axons were developmentally and intrinsically regulated. Indeed, the spontaneous activity of thalamic neurons governed axon growth and extension through the cortex in vivo. This activity-dependent modulation of growth was mediated by transcriptional regulation of Robo1 through an NF-κB binding site. Disruption of either the Robo1 or Slit1 genes accelerated the progression of thalamocortical axons in vivo, and interfering with Robo1 signaling restored normal axon growth in electrically silent neurons. Thus, modifications to spontaneous calcium activity encode a switch in the axon outgrowth program that allows the establishment of specific neuronal connections through the transcriptional regulation of Slit1 and Robo1 signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
López-Bendito, G. et al. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell 125, 127–142 (2006).
Métin, C., Deleglise, D., Serafini, T., Kennedy, T.E. & Tessier-Lavigne, M. A role for netrin-1 in the guidance of cortical efferents. Development 124, 5063–5074 (1997).
Garel, S. & Rubenstein, J.L.R. Intermediate targets in formation of topographic projections: inputs from the thalamocortical system. Trends Neurosci. 27, 533–539 (2004).
Braisted, J.E. et al. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J. Neurosci. 20, 5792–5801 (2000).
Tucker, K.L., Meyer, M. & Barde, Y. Neurotrophins are required for nerve growth during development. Nat. Neurosci. 4, 29–37 (2001).
Blackmore, M. & Letourneau, P.C. Changes within maturing neurons limit axonal regeneration in the developing spinal cord. J. Neurobiol. 66, 348–360 (2006).
Filbin, M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 4, 703–713 (2003).
Spitzer, N.C. Electrical activity in early neuronal development. Nature 444, 707–712 (2006).
Ming, G., Henley, J., Tessier-Lavigne, M., Song, H. & Poo, M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron 29, 441–452 (2001).
Goldberg, J.L. et al. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron 33, 689–702 (2002).
Fields, R.D., Neale, E. & Nelson, P. Effects of patterned electrical activity on neurite outgrowth from mouse sensory neurons. J. Neurosci. 10, 2950–2964 (1990).
Gomez, T.M. & Zheng, J.Q. The molecular basis for calcium-dependent axon pathfinding. Nat. Rev. Neurosci. 7, 115–125 (2006).
Tang, F., Dent, E.W. & Kalil, K. Spontaneous calcium transients in developing cortical neurons regulate axon outgrowth. J. Neurosci. 23, 927–936 (2003).
Cohan, C.S. & Kater, S. Suppression of neurite elongation and growth cone motility by electrical activity. Science 232, 1638–1640 (1986).
Borodinsky, L.N. et al. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429, 523–530 (2004).
Hanson, M.G. & Landmesser, L.T. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron 43, 687–701 (2004).
Marek, K.W., Kurtz, L. & Spitzer, N. cJun integrates calcium activity and tlx3 expression to regulate neurotransmitter specification. Nat. Neurosci. 13, 944–950 (2010).
López-Bendito, G. & Molnár, Z. Thalamocortical development: how are we going to get there? Nat. Rev. Neurosci. 4, 276–289 (2003).
Skaliora, I., Adams, R. & Blakemore, C. Morphology and growth patterns of developing thalamocortical axons. J. Neurosci. 20, 3650–3662 (2000).
Gomez, T.M. & Spitzer, N.C. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature 397, 350 (1999).
Burrone, J., O'Byrne, M. & Murthy, V.N. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature 420, 414–418 (2002).
Demarque, M. & Spitzer, N.C. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron 67, 321–334 (2010).
Mizuno, H., Hirano, T. & Tagawa, Y. Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. J. Neurosci. 27, 6760–6770 (2007).
Itoh, K., Ozaki, M., Stevens, B. & Fields, R. Activity-dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J. Neurobiol. 33, 735–748 (1997).
Dolmetsch, R.E., Lewis, R.S., Goodnow, C.C. & Healy, J.I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386, 855–858 (1997).
Fajardo, O., Meseguer, V., Belmonte, C. & Viana, F. TRPA1 channels: novel targets of 1,4-dihydropyridines. Channels (Austin) 2, 429–438 (2008).
Sugiyama, C. et al. Activator protein-1 responsive to the group II metabotropic glutamate receptor subtype in association with intracellular calcium in cultured rat cortical neurons. Neurochem. Int. 51, 467–475 (2007).
Xiang, G. et al. Identification of activity-dependent gene expression profiles reveals specific subsets of genes induced by different routes of Ca2+ entry in cultured rat cortical neurons. J. Cell. Physiol. 212, 126–136 (2007).
Bagri, A. et al. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 33, 233–248 (2002).
Stein, E. & Tessier-Lavigne, M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291, 1928–1938 (2001).
Sann, S.B., Xu, L., Nishimune, H., Sanes, J.R. & Spitzer, N.C. Neurite outgrowth and in vivo sensory innervation mediated by a Ca(V)2.2-laminin beta 2 stop signal. J. Neurosci. 28, 2366–2374 (2008).
Porter, B.E., Weis, J. & Sanes, J.R. A motoneuron-selective stop signal in the synaptic protein S-laminin. Neuron 14, 549–559 (1995).
Butler, A.K., Dantzker, J., Shah, R. & Callaway, E. Development of visual cortical axons: layer-specific effects of extrinsic influences and activity blockade. J. Comp. Neurol. 430, 321–331 (2001).
Uesaka, N., Hayano, Y., Yamada, A. & Yamamoto, N. Interplay between laminar specificity and activity-dependent mechanisms of thalamocortical axon branching. J. Neurosci. 27, 5215–5223 (2007).
Nishimaru, H., Iizuka, M., Ozaki, S. & Kudo, N. Spontaneous motoneuronal activity mediated by glycine and GABA in the spinal cord of rat fetuses in vitro. J. Physiol. (Lond.) 497, 131–143 (1996).
Scain, A.L. et al. Glycine release from radial cells modulates the spontaneous activity and its propagation during early spinal cord development. J. Neurosci. 30, 390–403 (2010).
Picken Bahrey, H.L. & Moody, W.J. Early development of voltage-gated ion currents and firing properties in neurons of the mouse cerebral cortex. J. Neurophysiol. 89, 1761–1773 (2003).
Xiao, Q., Xu, L. & Spitzer, N.C. Target-dependent regulation of neurotransmitter specification and embryonic neuronal calcium spike activity. J. Neurosci. 30, 5792–5801 (2010).
Ibarretxe, G., Perrais, D., Jaskolski, F., Vimeney, A. & Mulle, C. Fast regulation of axonal growth cone motility by electrical activity. J. Neurosci. 27, 7684–7695 (2007).
Gutierrez, H. & Davies, A.M. Regulation of neural process growth, elaboration and structural plasticity by NF-kappaB. Trends Neurosci. 34, 316–325 (2011).
Gutierrez, H., O'Keeffe, G.W., Gavalda, N., Gallagher, D. & Davies, A.M. Nuclear factor kappa B signaling either stimulates or inhibits neurite growth depending on the phosphorylation status of p65/RelA. J. Neurosci. 28, 8246–8256 (2008).
Riquelme, D. et al. High-frequency field stimulation of primary neurons enhances ryanodine receptor–mediated Ca2+ release and generates hydrogen peroxide, which jointly stimulate NF-kappaB activity. Antioxid. Redox Signal. 14, 1245–1259 (2011).
Tcherkezian, J., Brittis, P.A., Thomas, F., Roux, P.P. & Flanagan, J.G. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell 141, 632–644 (2010).
Jung, H., Yoon, B.C. & Holt, C.E. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat. Rev. Neurosci. 13, 308–324 (2012).
Andrews, W. et al. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development 133, 2243–2252 (2006).
López-Bendito, G. et al. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J. Neurosci. 27, 3395–3407 (2007).
Zallen, J.A., Yi, B.A. & Bargmann, C.I. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell 92, 217–227 (1998).
Hadjantonakis, A.K., Gertsenstein, M., Ikawa, M., Okabe, M. & Nagy, A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech. Dev. 76, 79–90 (1998).
Andrews, W. et al. The role of Slit-Robo signaling in the generation, migration and morphological differentiation of cortical interneurons. Dev. Biol. 313, 648–658 (2008).
Long, H. et al. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 42, 213–223 (2004).
Agmon, A. & Connors, B.W. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41, 365–379 (1991).
Acknowledgements
We thank N. García and E. San Martin for outstanding technical assistance. We are also grateful to A. Nagy (Samuel Lunenfeld Research Institute) for the EGFP mice, M. Valdeolmillos (Instituto de Neurociencias de Alicante) for providing the calcium imaging set-up and helping with quantification of calcium activity in growth cones, F. Martini for advice on calcium imaging, G. Expósito for advice on two-photon imaging, O. Marín (Instituto de Neurociencias de Alicante) for reagents, M. Maravall for advice on data analysis, F. Viana (Instituto de Neurociencias de Alicante) for providing the inducible CHO-A1 cells, and M. Dominguez and A. Gontijo for access and advice on qPCR equipment. We are grateful to A. Nieto, P. Arlotta, E. Herrera and M. Valdeolmillos for advice and critical reading of the manuscript. We are also grateful to members of the López-Bendito laboratory and Herrera laboratory for stimulating discussions and comments. C.M. is recipient of a JAE-Predoctoral Fellowship from the CSIC. This work was supported by grants from the Spanish Ministerio de Ciencia e Innovacion (BFU2006-07138 to J.L. and BFU2009-08261 to G.L.-B.), an Human Frontier Science Program Organization grant (RGP29/2008), the Consolider programme (CSD2007-00023) and an European Research Council grant (ERC-2009-StG_20081210 to G.L.-B.).
Author information
Authors and Affiliations
Contributions
G.L.-B. conceived the idea. E.M. and G.L.-B. designed the study. E.M., C.M., E.L.-D. and G.L.-B. performed the in vitro, ex vivo and in utero electroporation experiments. E.L.-D. and L.B. performed the luciferase assays. A.V.P. performed the electrophysiological recordings. P.S. and S.G. performed the experiments on Slit1 mutant mice. M.C.-P. performed the semiquantitative PCR and the collapse assay. M.J.L. performed the calcium recordings in CHO-A1 cells. S.P. subcloned the Kir2.1 plasmid. M.T.-L. produced the Robo1, Robo2 and Robo1; Robo2 mutant mice. J.G. supervised the luciferase assays. J.L. supervised the electrophysiological experiments. E.M., C.M. and G.L.-B. conducted the data analysis and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 16642 kb)
Supplementary Video 1
Speed of growth of TCA travelling at the vTel. (AVI 4100 kb)
Supplementary Video 2
Speed of growth of TCA extending at the neocortex. (AVI 6660 kb)
Supplementary Video 3
Speed of growth of TCA travelling at the angle at the PSPB. (AVI 3588 kb)
Supplementary Video 4
Speed of growth of TCA travelling at the entrance of the neocortex. (AVI 1077 kb)
Supplementary Video 5
Spontaneous activity in the thalamus of E12.5 embryo. (AVI 5969 kb)
Supplementary Video 6
Spontaneous activity in the thalamus of E16.5 embryo. (AVI 7184 kb)
Supplementary Video 7
Spontaneous activity in early thalamocortical growth cones. (AVI 4007 kb)
Supplementary Video 8
Spontaneous activity in late thalamocortical growth cones. (AVI 3428 kb)
Rights and permissions
About this article
Cite this article
Mire, E., Mezzera, C., Leyva-Díaz, E. et al. Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth. Nat Neurosci 15, 1134–1143 (2012). https://doi.org/10.1038/nn.3160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3160
This article is cited by
-
Development of Auditory Cortex Circuits
Journal of the Association for Research in Otolaryngology (2021)
-
Prenatal thalamic waves regulate cortical area size prior to sensory processing
Nature Communications (2017)
-
Fate and freedom in developing neocortical circuits
Nature Communications (2017)
-
Genetic and activity-dependent mechanisms underlying interneuron diversity
Nature Reviews Neuroscience (2017)