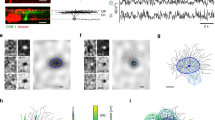
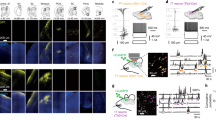
Abstract
Transforming synaptic input into action potential output is a fundamental function of neurons. The pattern of action potential output from principal cells of the mammalian hippocampus encodes spatial and nonspatial information, but the cellular and circuit mechanisms by which neurons transform their synaptic input into a given output are unknown. Using a combination of optical activation and cell type–specific pharmacogenetic silencing in vitro, we found that dendritic inhibition is the primary regulator of input-output transformations in mouse hippocampal CA1 pyramidal cells, and acts by gating the dendritic electrogenesis driving burst spiking. Dendrite-targeting interneurons are themselves modulated by interneurons targeting pyramidal cell somata, providing a synaptic substrate for tuning pyramidal cell output through interactions in the local inhibitory network. These results provide evidence for a division of labor in cortical circuits, where distinct computational functions are implemented by subtypes of local inhibitory neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kandel, E.R. & Spencer, W.A. Electrophysiology of hippocampal neurons II: after-potentials and repetitive firing. J. Neurophysiol. 24, 243–259 (1961).
Thompson, L.T. & Best, P.J. Place cells and silent cells in the hippocampus of freely behaving rats. J. Neurosci. 9, 2382–2390 (1989).
O'Keefe, J. & Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971).
O'Keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map (Oxford University Press, Oxford, 1978).
Ahmed, O.J. & Mehta, M.R. The hippocampal rate code: anatomy, physiology, and theory. Trends Neurosci. 32, 329–338 (2009).
Colgin, L.L., Moser, E.I. & Moser, M.-B. Understanding memory through hippocampal remapping. Trends Neurosci. 31, 469–477 (2008).
Nevian, T., Larkum, M.E., Polsky, A. & Schiller, J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat. Neurosci. 10, 206–214 (2007).
Larkum, M.E., Zhu, J.J. & Sakmann, B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 398, 338–341 (1999).
Gasparini, S. & Magee, J.C. State-dependent dendritic computation in hippocampal CA1 pyramidal neurons. J. Neurosci. 26, 2088–2100 (2006).
Losonczy, A. & Magee, J.C. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron 50, 291–307 (2006).
Harvey, C.D., Collman, F., Dombeck, D.A. & Tank, D.W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946 (2009).
Epsztein, J., Brecht, M. & Lee, A.K. Intracellular determinants of hippocampal CA1 place and silence cell activity in a novel environment. Neuron 70, 109–120 (2011).
Isaacson, J.S. & Scanziani, M. How inhibition shapes cortical activity. Neuron 72, 231–243 (2011).
Buhl, E.H., Halasy, K. & Somogyi, P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 368, 823–828 (1994).
Freund, T.F. & Buzsáki, G. Interneurons of the hippocampus. Hippocampus 6, 347–470 (1996).
Klausberger, T. & Somogyi, P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57 (2008).
Cobb, S.R., Buhl, E.H., Halasy, K., Paulsen, O. & Somogyi, P. Synchronization of neuronal activity in hippocampus by individual GABAergic neurons. Nature 378, 75–78 (1995).
Pouille, F. & Scanziani, M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163 (2001).
Losonczy, A., Zemelman, B.V., Vaziri, A. & Magee, J.C. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 13, 967–972 (2010).
Megías, M., Emri, Z., Freund, T.F. & Gulyas, A.I. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102, 527–540 (2001).
Klausberger, T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur. J. Neurosci. 30, 947–957 (2009).
Klausberger, T. et al. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat. Neurosci. 7, 41–47 (2004).
Murayama, M. et al. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 457, 1137–1141 (2009).
Larkum, M.E., Nevian, T., Sandler, M., Polsky, A. & Schiller, J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 325, 756–760 (2009).
Miles, R., Tóth, K., Gulyás, A.I., Hájos, N. & Freund, T.F. Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16, 815–823 (1996).
Collingridge, G.L., Herron, C.E. & Lester, R.A.J. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral–commissural pathway of rat hippocampus. J. Physiol. (Lond.) 399, 282–300 (1988).
Poirazi, P., Brannon, T. & Mel, B.W. Pyramidal neuron as 2-layer neural network. Neuron 37, 989–999 (2003).
Losonczy, A., Makara, J.K. & Magee, J.C. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature 452, 436–441 (2008).
Branco, T. & Häusser, M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr. Opin. Neurobiol. 20, 494–502 (2010).
Rial Verde, E.M., Zayat, L., Etchenique, R. & Yuste, R. Photorelease of GABA with visible light using an inorganic caging group. Front. Neural Circuits 2, 2 (2008).
Williams, S.R. Spatial compartmentalization and functional impact of conductance in pyramidal neurons. Nat. Neurosci. 7, 961–967 (2004).
Williams, S.R. & Stuart, G.J. Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science 295, 1907–1910 (2002).
Knowles, W.D. & Schwartzkroin, P.S. Axonal ramifications of hippocampal CA1 pyramidal cells. J. Neurosci. 1, 1236–1241 (1981).
Buzsáki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 2002).
Magnus, C.J. et al. Chemical and genetic engineering of selective ligand-ion channel interactions. Science 333, 1292–1296 (2011).
Atasoy, D., Aponte, Y., Su, H.H. & Sternson, S.M.A. FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008).
Losonczy, A., Zhang, L., Shigemoto, R., Somogyi, P. & Nusser, Z. Cell type dependence and variability in the short-term plasticity of EPSCs in identified mouse hippocampal interneurons. J. Physiol. (Lond.) 542, 193–210 (2002).
Klausberger, T. et al. Brain state– and cell type–specific firing of hippocampal interneurons in vivo. Nature 421, 844–848 (2003).
Katona, I. et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 19, 4544–4558 (1999).
Magee, J.C. & Carruth, M. Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J. Neurophysiol. 82, 1895–1901 (1999).
Takahashi, H. & Magee, J.C. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron 62, 102–111 (2009).
Golding, N.L., Jung, H.Y., Mickus, T. & Spruston, N. Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. J. Neurosci. 19, 8789–8798 (1999).
Maccaferri, G., Roberts, J.D., Szucs, P., Cottingham, C.A. & Somogyi, P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J. Physiol. (Lond.) 524, 91–116 (2000).
Cobb, S.R. et al. Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience 79, 629–648 (1997).
Jarsky, T., Roxin, A., Kath, W.L. & Spruston, N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat. Neurosci. 8, 1667–1676 (2005).
Mulligan, K.A. & Törk, I. Serotoninergic innervation of the cat cerebral cortex. J. Comp. Neurol. 270, 86–110 (1988).
Varga, V. et al. Fast synaptic subcortical control of hippocampal circuits. Science 326, 449–453 (2009).
Cope, D.W. et al. Cholecystokinin-immunopositive basket and Schaffer collateral–associated interneurons target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience 109, 63–80 (2002).
Fuentealba, P. et al. Ivy cells: a population of nitric oxide–producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron 57, 917–929 (2008).
Poirazi, P., Brannon, T. & Mel, B.W. Arithmetic of subthreshold synaptic summation in a model CA1 pyramidal cell. Neuron 37, 977–987 (2003).
Acknowledgements
We thank R. Nyilas, R. Field, A. Qureshi, J.K. Baruni and A. Villacis for help with histology. We thank J.C. Magee, L.F. Abbott, G. Buzsáki, S.A. Siegelbaum and T.M. Jessell for discussions and comments on a previous version of the manuscript. This work was supported by Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarships (M.L.-B. and P.K.), the Searle Scholar Program, the Gatsby Foundation and Kavli Institute at Columbia University (A.L.) and the Howard Hughes Medical Institute (S.M.S.). F.B. thanks the Agence Nationale pour la Recherche (France) for financial support (ANR PCV 07 10035).
Author information
Authors and Affiliations
Contributions
M.L.-B. and A.L. designed the study. M.L.-B., G.F.T. and A.L. performed all electrophysiological and anatomical experiments and analyzed the data. P.K. performed compartmental modeling. P.H.L. and S.M.S. provided constructs and compounds for the PSAM-PSEM method. F.B., X.-H.S. and J.F.N. synthesized and provided PENB-L-glutamate. B.V.Z. prepared plasmids, designed rAAV viruses and generated knock-in mouse lines. M.L.-B. and A.L. wrote the paper with the help of the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 9685 kb)
Rights and permissions
About this article
Cite this article
Lovett-Barron, M., Turi, G., Kaifosh, P. et al. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci 15, 423–430 (2012). https://doi.org/10.1038/nn.3024
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3024
This article is cited by
-
mTORC1-mediated acquisition of reward-related representations by hippocampal somatostatin interneurons
Molecular Brain (2023)
-
Stimulation of protein synthesis by optogenetic and chemical induction of excitatory synaptic plasticity in hippocampal somatostatin interneurons
Molecular Brain (2022)
-
The role of inhibitory circuits in hippocampal memory processing
Nature Reviews Neuroscience (2022)
-
Neuropeptides and small-molecule amine transmitters: cooperative signaling in the nervous system
Cellular and Molecular Life Sciences (2022)
-
Somatostatin contributes to long-term potentiation at excitatory synapses onto hippocampal somatostatinergic interneurons
Molecular Brain (2021)