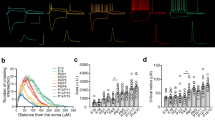
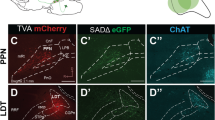
Abstract
Neostriatal cholinergic interneurons are believed to be important for reinforcement-mediated learning and response selection by signaling the occurrence and motivational value of behaviorally relevant stimuli through precisely timed multiphasic population responses. An important problem is to understand how these signals regulate the functioning of the neostriatum. Here we describe the synaptic organization of a previously unknown circuit that involves direct nicotinic excitation of several classes of GABAergic interneurons, including neuroptide Y–expressing neurogilaform neurons, and enables cholinergic interneurons to exert rapid inhibitory control of the activity of projection neurons. We also found that, in vivo, the dominant effect of an optogenetically reproduced pause-excitation population response of cholinergic interneurons was powerful and rapid inhibition of the firing of projection neurons that is coincident with synchronous cholinergic activation. These results reveal a previously unknown circuit mechanism that transmits reinforcement-related information of ChAT interneurons in the mouse neostriatal network.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Graybiel, A.M. The basal ganglia and chunking of action repertoires. Neurobiol. Learn. Mem. 70, 119–136 (1998).
Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27 (1998).
Morris, G., Arkadir, D., Nevet, A., Vaadia, E. & Bergman, H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 43, 133–143 (2004).
Joshua, M., Adler, A., Mitelman, R., Vaadia, E. & Bergman, H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J. Neurosci. 28, 11673–11684 (2008).
Hyland, B.I., Reynolds, J.N., Hay, J., Perk, C.G. & Miller, R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience 114, 475–492 (2002).
Kimura, M., Rajkowski, J. & Evarts, E. Tonically discharging putamen neurons exhibit set-dependent responses. Proc. Natl. Acad. Sci. USA 81, 4998–5001 (1984).
Apicella, P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 30, 299–306 (2007).
Aosaki, T. et al. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J. Neurosci. 14, 3969–3984 (1994).
Aosaki, T., Kimura, M. & Graybiel, A.M. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J. Neurophysiol. 73, 1234–1252 (1995).
Tecuapetla, F., Koos, T., Tepper, J.M., Kabbani, N. & Yeckel, M.F. Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J. Neurosci. 29, 8977–8990 (2009).
Pearce, R.A. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron 10, 189–200 (1993).
Banks, M.I., Li, T.B. & Pearce, R.A. The synaptic basis of GABAA,slow . J. Neurosci. 18, 1305–1317 (1998).
Tamás, G., Lorincz, A., Simon, A. & Szabadics, J. Identified sources and targets of slow inhibition in the neocortex. Science 299, 1902–1905 (2003).
Karayannis, T. et al. Slow GABA transient and receptor desensitization shape synaptic responses evoked by hippocampal neurogliaform cells. J. Neurosci. 30, 9898–9909 (2010).
Szabadics, J., Tamas, G. & Soltesz, I. Different transmitter transients underlie presynaptic cell type specificity of GABAA,slow and GABAA,fast . Proc. Natl. Acad. Sci. USA 104, 14831–14836 (2007).
Banks, M.I., White, J.A. & Pearce, R.A. Interactions between distinct GABAA circuits in hippocampus. Neuron 25, 449–457 (2000).
Overstreet, L.S., Jones, M.V. & Westbrook, G.L. Slow desensitization regulates the availability of synaptic GABAA receptors. J. Neurosci. 20, 7914–7921 (2000).
Ibáñez-Sandoval, O. et al. A novel functionally distinct subtype of striatal neuropeptide Y interneuron. J. Neurosci. 31, 16757–16769 (2011).
Gittis, A.H., Nelson, A.B., Thwin, M.T., Palop, J.J. & Kreitzer, A.C. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J. Neurosci. 30, 2223–2234 (2010).
Capogna, M. & Pearce, R.A. GABAA,slow: causes and consequences. Trends Neurosci. 34, 101–112 (2011).
Hill, J.A. Jr., Zoli, M., Bourgeois, J.P. & Changeux, J.P. Immunocytochemical localization of a neuronal nicotinic receptor: the beta 2-subunit. J. Neurosci. 13, 1551–1568 (1993).
Koós, T. & Tepper, J.M. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci. 2, 467–472 (1999).
Koós, T. & Tepper, J.M. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J. Neurosci. 22, 529–535 (2002).
Chang, H.T. & Kita, H. Interneurons in the rat striatum: relationships between parvalbumin neurons and cholinergic neurons. Brain Res. 574, 307–311 (1992).
Sullivan, M.A., Chen, H. & Morikawa, H. Recurrent inhibitory network among striatal cholinergic interneurons. J. Neurosci. 28, 8682–8690 (2008).
Ding, J.B., Guzman, J.N., Peterson, J.D., Goldberg, J.A. & Surmeier, D.J. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 67, 294–307 (2010).
Giniatullin, R., Nistri, A. & Yakel, J.L. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 28, 371–378 (2005).
Wilson, C.J. The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron 45, 575–585 (2005).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Apicella, P., Ravel, S., Sardo, P. & Legallet, E. Influence of predictive information on responses of tonically active neurons in the monkey striatum. J. Neurophysiol. 80, 3341–3344 (1998).
Gradinaru, V., Thompson, K.R. & Deisseroth, K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 36, 129–139 (2008).
Witten, I.B. et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330, 1677–1681 (2010).
Oláh, S. et al. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461, 1278–1281 (2009).
Banks, M.I. & Pearce, R.A. Kinetic differences between synaptic and extrasynaptic GABA(A) receptors in CA1 pyramidal cells. J. Neurosci. 20, 937–948 (2000).
Wonnacott, S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 20, 92–98 (1997).
McGehee, D.S., Heath, M.J., Gelber, S., Devay, P. & Role, L.W. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 269, 1692–1696 (1995).
De Rover, M., Lodder, J.C., Schoffelmeer, A.N. & Brussaard, A.B. Intermittent morphine treatment induces a long-lasting increase in cholinergic modulation of GABAergic synapses in nucleus accumbens of adult rats. Synapse 55, 17–25 (2005).
Kubota, Y., Mikawa, S. & Kawaguchi, Y. Neostriatal GABAergic interneurones contain NOS, calretinin or parvalbumin. Neuroreport 5, 205–208 (1993).
Ibáñez-Sandoval, O. et al. Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase–expressing neurons in adult mouse striatum. J. Neurosci. 30, 6999–7016 (2010).
Berke, J.D. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur. J. Neurosci. 30, 848–859 (2009).
Zhou, F.M., Liang, Y. & Dani, J.A. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 4, 1224–1229 (2001).
Rice, M.E. & Cragg, S.J. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 7, 583–584 (2004).
Matsumoto, N., Minamimoto, T., Graybiel, A.M. & Kimura, M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J. Neurophysiol. 85, 960–976 (2001).
Hikosaka, O., Sakamoto, M. & Usui, S. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J. Neurophysiol. 61, 814–832 (1989).
Kataoka, Y. et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J. Comp. Neurol. 518, 277–291 (2010).
Leckman, J.F., Vaccarino, F.M., Kalanithi, P.S. & Rothenberger, A. Annotation: Tourette syndrome: a relentless drumbeat driven by misguided brain oscillations. J. Child Psychol. Psychiatry 47, 537–550 (2006).
Han, X. et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron 62, 191–198 (2009).
Yáñez-Muñoz, R.J. et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 12, 348–353 (2006).
Jog, M.S. et al. Tetrode technology: advances in implantable hardware, neuroimaging, and data analysis techniques. J. Neurosci. Methods 117, 141–152 (2002).
Berke, J.D. Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. J. Neurosci. 28, 10075–10080 (2008).
Acknowledgements
We thank J. Berlin for confocal microscopy, L. Zaborszky for providing ChAT-EGFP mice, R. Yanez-Munoz for providing integration deficient pCMV-dR8.74-D64V plasmid DNA and for advice regarding virus production, N. Altan-Bonnet, W. Friedman and Haesun Kim for generously providing access to an ultracentrifuge facility and other equipment in their laboratories, C.T. Unal and A. Kreitzer for valuable discussion, A. Berenyi, S. Fujisawa and M. Vandecasteele for advice regarding in vivo recording methods, H. Xenias for help with confocal imaging, F. Shah for help with immunocytochemical procedures and other technical assistance, and I. Tadros for virus injections. The research was supported by US National Institutes of Health grant NS072950 and a Busch Biomedical Research Grant of Rutgers University to T.K., US National Institutes of Health grant NS034865 to J.M.T. and Rutgers University funds.
Author information
Authors and Affiliations
Contributions
D.F.E. carried out all of the in vivo recording experiments and data analysis, performed the majority of the in vitro experiments, and contributed to virus production, virus injections and confocal imaging (with the exception of Fig. 1a, which was produced by J. Berlin). O.I.-S. and F.T. performed the initial in vitro analysis of NPY-NGF neurons and O.I.-S. identified nicotinic synapses in these interneurons. E.S. contributed to the design of in vivo recording, optical stimulation methods and data analysis, molecular biology, and virus production. G.B. contributed to optrode design and the design and analysis of in vivo recording experiments. K.D. designed and provided constructs for optogenetic expression vectors, designed and produced the AAV5-DIO-eNpHR3.0-YFP and the AAV5-DIO-ChR2-mCherry virus vectors, and contributed to optogenetic methods. J.M.T. contributed to the development of in vitro and in vivo recording methods. T.K. performed in vitro recordings, recombinant DNA procedures and lentivirus production. The study was designed by T.K., J.M.T. and D.F.E. and the manuscript was written by T.K. with substantial contributions from D.F.E. and J.M.T. and input from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 1356 kb)
Rights and permissions
About this article
Cite this article
English, D., Ibanez-Sandoval, O., Stark, E. et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci 15, 123–130 (2012). https://doi.org/10.1038/nn.2984
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2984
This article is cited by
-
The early excitatory action of striatal cholinergic-GABAergic microcircuits conditions the subsequent GABA inhibitory shift
Communications Biology (2023)
-
Electrophysiological insights into deep brain stimulation of the network disorder dystonia
Pflügers Archiv - European Journal of Physiology (2023)
-
A spiking computational model for striatal cholinergic interneurons
Brain Structure and Function (2023)
-
Dopamine D2 receptors modulate the cholinergic pause and inhibitory learning
Molecular Psychiatry (2022)
-
Oxycodone in the Opioid Epidemic: High ‘Liking’, ‘Wanting’, and Abuse Liability
Cellular and Molecular Neurobiology (2021)