Abstract
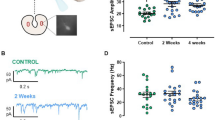
Long-term synaptic changes, which are essential for learning and memory, are dependent on homeostatic mechanisms that stabilize neural activity. Homeostatic responses have also been implicated in pathological conditions, including nicotine addiction. Although multiple homeostatic pathways have been described, little is known about how compensatory responses are tuned to prevent them from overshooting their optimal range of activity. We found that prolonged inhibition of nicotinic acetylcholine receptors (nAChRs), the major excitatory receptors in the Drosophila CNS, resulted in a homeostatic increase in the Drosophila α7 (Dα7)-nAChR. This response then induced an increase in the transient A-type K+ current carried by Shaker cognate L (Shal; also known as voltage-gated K+ channel 4, Kv4) channels. Although increasing Dα7-nAChRs boosted miniature excitatory postsynaptic currents, the ensuing increase in Shal channels served to stabilize postsynaptic potentials. These data identify a previously unknown mechanism for fine tuning the homeostatic response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Turrigiano, G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci. 34, 89–103 (2011).
Davis, G.W. Homeostatic control of neural activity: from phenomenology to molecular design. Annu. Rev. Neurosci. 29, 307–323 (2006).
Pozo, K. & Goda, Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66, 337–351 (2010).
Desai, N.S., Rutherford, L.C. & Turrigiano, G.G. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat. Neurosci. 2, 515–520 (1999).
Kuba, H., Oichi, Y. & Ohmori, H. Presynaptic activity regulates Na+ channel distribution at the axon initial segment. Nature 465, 1075–1078 (2010).
Baines, R.A., Uhler, J.P., Thompson, A., Sweeney, S.T. & Bate, M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21, 1523–1531 (2001).
Nataraj, K., Le Roux, N., Nahmani, M., Lefort, S. & Turrigiano, G. Visual deprivation suppresses L5 pyramidal neuron excitability by preventing the induction of intrinsic plasticity. Neuron 68, 750–762 (2010).
De Biasi, M. & Dani, J.A. Reward, addiction, withdrawal to nicotine. Annu. Rev. Neurosci. 34, 105–130 (2011).
Picciotto, M.R., Addy, N.A., Mineur, Y.S. & Brunzell, D.H. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog. Neurobiol. 84, 329–342 (2008).
Fenster, C.P., Whitworth, T.L., Sheffield, E.B., Quick, M.W. & Lester, R.A. Upregulation of surface α4β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J. Neurosci. 19, 4804–4814 (1999).
Tsunoda, S. & Salkoff, L. The major delayed rectifier in both Drosophila neurons and muscle is encoded by Shab. J. Neurosci. 15, 5209–5221 (1995b).
Tsunoda, S. & Salkoff, L. Genetic analysis of Drosophila neurons: Shal, Shaw, and Shab encode most embryonic potassium currents. J. Neurosci. 15, 1741–1754 (1995a).
Lee, D. & O'Dowd, D.K. Fast excitatory synaptic transmission mediated by nicotinic acetylcholine receptors in Drosophila neurons. J. Neurosci. 19, 5311–5321 (1999).
Leung, H.T., Branton, W.D., Phillips, H.S., Jan, L. & Byerly, L. Spider toxins selectively block calcium currents in Drosophila. Neuron 3, 767–772 (1989).
Ping, Y. et al. Shal/Kv4 channels are required for maintaining excitability during repetitive firing and normal locomotion in Drosophila. PLoS ONE 6, e16043 (2011).
Su, H. & O'Dowd, D.K. Fast synaptic currents in Drosophila mushroom body Kenyon cells are mediated by α-bungarotoxin-sensitive nicotinic acetylcholine receptors and picrotoxin-sensitive GABA receptors. J. Neurosci. 23, 9246–9253 (2003).
Schmidt, H. et al. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev. Biol. 189, 186–204 (1997).
Gotti, C. et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol. 78, 703–711 (2009).
Albuquerque, E.X., Pereira, E.F., Alkondon, M. & Rogers, S.W. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 89, 73–120 (2009).
Aracava, Y., Pereira, E.F., Maelicke, A. & Albuquerque, E.X. Memantine blocks α7* nicotinic acetylcholine receptors more potently than N-methyl-D-aspartate receptors in rat hippocampal neurons. J. Pharmacol. Exp. Ther. 312, 1195–1205 (2005).
Brickley, S.G., Revilla, V., Cull–Candy, S.G., Wisden, W. & Farrant, M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409, 88–92 (2001).
Pulver, S.R. & Griffith, L.C. Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Nat. Neurosci. 13, 53–59 (2010).
Grauso, M., Reenan, R.A., Culetto, E. & Sattelle, D.B. Novel putative nicotinic acetylcholine receptor subunit genes, Dα5, Dα6 and Dα7, in Drosophila melanogaster identify a new and highly conserved target of adenosine deaminase acting on RNA-mediated A-to-I pre-mRNA editing. Genetics 160, 1519–1533 (2002).
Griffith, L.C. et al. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron 10, 501–509 (1993).
Tripodi, M., Evers, J.F., Mauss, A., Bate, M. & Landgraf, M. Structural homeostasis: compensatory adjustments of dendritic arbor geometry in response to variations of synaptic input. PLoS Biol. 6, e260 (2008).
Kremer, M.C. et al. Structural long-term changes at mushroom body input synapses. Curr. Biol. 20, 1938–1944 (2010).
Bushey, D., Tononi, G. & Cirelli, C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science 332, 1576–1581 (2011).
Chou, Y.H. et al. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat. Neurosci. 13, 439–449 (2010).
Lichtman, J.W. & Colman, H. Synapse elimination and indelible memory. Neuron 25, 269–278 (2000).
Small, D.H. Network dysfunction in Alzheimer's disease: does synaptic scaling drive disease progression? Trends Mol. Med. 14, 103–108 (2008).
Jerng, H.H., Pfaffinger, P.J. & Covarrubias, M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol. Cell. Neurosci. 27, 343–369 (2004).
Chen, X. et al. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J. Neurosci. 26, 12143–12151 (2006).
Kim, J., Jung, S.C., Clemens, A.M., Petralia, R.S. & Hoffman, D.A. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron 54, 933–947 (2007).
Cai, X. et al. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44, 351–364 (2004).
Thiagarajan, T.C., Piedras–Renteria, E.S. & Tsien, R.W. α- and β-CaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron 36, 1103–1114 (2002).
Groth, R.D., Lindskog, M., Thiagarajan, T.C., Li, L. & Tsien, R.W. β Ca2+/CaM-dependent kinase type II triggers upregulation of GluA1 to coordinate adaptation to synaptic inactivity in hippocampal neurons. Proc. Natl. Acad. Sci. USA 108, 828–833 (2011).
Varga, A.W. et al. Calcium–calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J. Neurosci. 24, 3643–3654 (2004).
Misonou, H. Homeostatic regulation of neuronal excitability by K+ channels in normal and diseased brains. Neuroscientist 16, 51–64 (2010).
Magee, J.C. & Cook, E.P. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat. Neurosci. 3, 895–903 (2000).
Hoffman, D.A., Magee, J.C., Colbert, C.M. & Johnston, D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387, 869–875 (1997).
Kazama, H. & Wilson, R.I. Homeostatic matching and nonlinear amplification at identified central synapses. Neuron 58, 401–413 (2008).
Fujioka, M. et al. Even-skipped, acting as a repressor, regulates axonal projections in Drosophila. Development 130, 5385–5400 (2003).
Fayyazuddin, A., Zaheer, M.A., Hiesinger, P.R. & Bellen, H.J. The nicotinic acetylcholine receptor Dα7 is required for an escape behavior in Drosophila. PLoS Biol. 4, e63 (2006).
Watson, G.B. et al. A spinosyn–sensitive Drosophila melanogaster nicotinic acetylcholine receptor identified through chemically induced target site resistance, resistance gene identification, and heterologous expression. Insect Biochem. Mol. Biol. 40, 376–384 (2010).
Hegde, P., Gu, G.G., Chen, D., Free, S.J. & Singh, S. Mutational analysis of the Shab-encoded delayed rectifier K(+) channels in Drosophila. J. Biol. Chem. 274, 22109–22113 (1999).
Diao, F., Waro, G. & Tsunoda, S. Fast inactivation of Shal (Kv4) K+ channels is regulated by the novel interactor SKIP3 in Drosophila neurons. Mol. Cell. Neurosci. 42, 33–44 (2009).
Schulz, R. et al. Neuronal nicotinic acetylcholine receptors from Drosophila: two different types of alpha subunits coassemble within the same receptor complex. J. Neurochem. 74, 2537–2546 (2000).
Jonas, P.E., Phannavong, B., Schuster, R., Schroder, C. & Gundelfinger, E.D. Expression of the ligand-binding nicotinic acetylcholine receptor subunit Dα2 in the Drosophila central nervous system. J. Neurobiol. 25, 1494–1508 (1994).
Chamaon, K., Schulz, R., Smalla, K.H., Seidel, B. & Gundelfinger, E.D. Neuronal nicotinic acetylcholine receptors of Drosophila melanogaster: the α-subunit Dα3 and the β-type subunit ARD co–assemble within the same receptor complex. FEBS Lett. 482, 189–192 (2000).
Chamaon, K., Smalla, K.H., Thomas, U. & Gundelfinger, E.D. Nicotinic acetylcholine receptors of Drosophila: three subunits encoded by genomically linked genes can co–assemble into the same receptor complex. J. Neurochem. 80, 149–157 (2002).
Acknowledgements
We thank G. Waro for genetic crosses and technical assistance. We thank H. Bellen (Baylor College of Medicine) for the antibody to Dα7 and the Dα7-deficient mutant line, S. Sigrist (Institute for Biology/Genetics) for the UAS–nAcRα-18C-EGFP fly line, M. Fujioka (Thomas Jefferson University) for the RRa-GAL4, RN2-GAL4 and EL-GAL4 fly lines, S. Singh (State University of New York, Buffalo) for the Shab[3] line, and E. Gundelfinger and U. Thomas (Leibniz Institute for Neurobiology) for the antibodies to Drosophila nAChRs. We also thank C. Yeung for carrying out genetic mapping and crosses for the transgenic HA-Shal line. S.T. is supported by a grant from the US National Institutes of Health (R01 GM083335).
Author information
Authors and Affiliations
Contributions
Y.P. conducted all of the experiments and analyzed all of the data. S.T. supervised the project and wrote the majority of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 843 kb)
Rights and permissions
About this article
Cite this article
Ping, Y., Tsunoda, S. Inactivity-induced increase in nAChRs upregulates Shal K+ channels to stabilize synaptic potentials. Nat Neurosci 15, 90–97 (2012). https://doi.org/10.1038/nn.2969
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2969
This article is cited by
-
ACh Transfers: Homeostatic Plasticity of Cholinergic Synapses
Cellular and Molecular Neurobiology (2023)
-
Differential gene expression in Drosophila melanogaster and D. nigrosparsa infected with the same Wolbachia strain
Scientific Reports (2021)
-
The mirtron miR-1010 functions in concert with its host gene SKIP to balance elevation of nAcRβ2
Scientific Reports (2020)
-
Distinct expression of potassium channels regulates visual response properties of lamina neurons in Drosophila melanogaster
Journal of Comparative Physiology A (2020)