Abstract
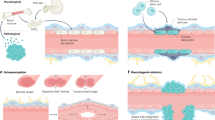
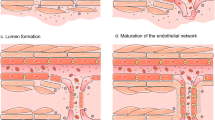
Blood vessels in the nervous system have traditionally been considered neutral bystanders that only passively adapt their structure and function in response to the needs of neural cells. Emerging evidence suggests, however, that vessels and angiogenic molecules actively participate in the pathogenesis of neurological disorders. Here we will discuss molecular insights into neurological disorders resulting either from excessive vessel growth (cerebral vascular malformations) or improper vessel regression (neurodegeneration and white matter lesions). These genetic insights offer alternative therapeutic options, some of which are being evaluated in the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carmeliet, P. & Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Winkler, E.A., Bell, R.D. & Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011).
San Millán Ruíz, D.S., Yilmaz, H. & Gailloud, P. Cerebral developmental venous anomalies: current concepts. Ann. Neurol. 66, 271–283 (2009).
Leblanc, G.G., Golanov, E., Awad, I.A. & Young, W.L. Biology of vascular malformations of the brain. Stroke 40, e694–e702 (2009).
Riant, F., Bergametti, F., Ayrignac, X., Boulday, G. & Tournier-Lasserve, E. Recent insights into cerebral cavernous malformations: the molecular genetics of CCM. FEBS J. 277, 1070–1075 (2010).
Hauptman, J.S., Moftakhar, P., Dadour, A., Malkasian, D. & Martin, N.A. Advances in the biology of cerebral cavernous malformations. Surg. Neurol. Int. 1, 63 (2010).
McDonald, D.A. et al. A novel mouse model of cerebral cavernous malformations based on the two-hit mutation hypothesis recapitulates the human disease. Hum. Mol. Genet. 20, 211–222 (2011).
Chan, A.C., Li, D.Y., Berg, M.J. & Whitehead, K.J. Recent insights into cerebral cavernous malformations: animal models of CCM and the human phenotype. FEBS J. 277, 1076–1083 (2010).
Cunningham, K. et al. Conditional deletion of Ccm2 causes hemorrhage in the adult brain: a mouse model of human cerebral cavernous malformations. Hum. Mol. Genet. 20, 3198–3206 (2011).
Shovlin, C.L. Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev. 24, 203–219 (2010).
Corti, P. et al. Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development 138, 1573–1582 (2011).
Park, S.O. et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J. Clin. Invest. 119, 3487–3496 (2009).
Gridley, T. Notch signaling in the vasculature. Curr. Top. Dev. Biol. 92, 277–309 (2010).
Gao, P. et al. Nonischemic cerebral venous hypertension promotes a pro-angiogenic stage through HIF-1 downstream genes and leukocyte-derived MMP-9. J. Cereb. Blood Flow Metab. 29, 1482–1490 (2009).
Kim, H. et al. Brain arteriovenous malformation biology relevant to hemorrhage and implication for therapeutic development. Stroke 40, S95–S97 (2009).
Dupuis-Girod, S., Bailly, S. & Plauchu, H. Hereditary hemorrhagic telangiectasia: from molecular biology to patient care. J. Thromb. Haemost. 8, 1447–1456 (2010).
Pardali, E., Goumans, M.J. & ten Dijke, P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 20, 556–567 (2010).
Ricard, N. et al. Functional analysis of the BMP9 response of ALK1 mutants from HHT2 patients: a diagnostic tool for novel ACVRL1 mutations. Blood 116, 1604–1612 (2010).
Walker, E.J. et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann. Neurol. 69, 954–962 (2011).
Boon, L.M., Mulliken, J.B. & Vikkula, M. RASA1: variable phenotype with capillary and arteriovenous malformations. Curr. Opin. Genet. Dev. 15, 265–269 (2005).
Anand, S. et al. MicroRNA-132–mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med. 16, 909–914 (2010).
Rizzo, M.T. & Leaver, H.A. Brain endothelial cell death: modes, signaling pathways, and relevance to neural development, homeostasis, and disease. Mol. Neurobiol. 42, 52–63 (2010).
Yamamoto, Y., Craggs, L., Baumann, M., Kalimo, H. & Kalaria, R.N. Review: molecular genetics and pathology of hereditary small vessel diseases of the brain. Neuropathol. Appl. Neurobiol. 37, 94–113 (2011).
Ayata, C. CADASIL: experimental insights from animal models. Stroke 41, S129–S134 (2010).
Assareh, A., Mather, K.A., Schofield, P.R., Kwok, J.B. & Sachdev, P.S. The genetics of white matter lesions. CNS Neurosci. Ther. published online, doi:10.1111/j.1755-5949.2010.00181.x (7 July 2010).
Lewandowska, E. et al. Capillary vessel wall in CADASIL angiopathy. Folia Neuropathol. 48, 104–115 (2010).
Chabriat, H., Joutel, A., Dichgans, M., Tournier-Lasserve, E. & Bousser, M.G. CADASIL. Lancet Neurol. 8, 643–653 (2009).
Joutel, A. et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J. Clin. Invest. 120, 433–445 (2010).
Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008).
Iadecola, C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 120, 287–296 (2010).
Haass, C. & Selkoe, D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 (2007).
Brun, A. & Englund, E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann. Neurol. 19, 253–262 (1986).
Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 5, 347–360 (2004).
Mawuenyega, K.G. et al. Decreased clearance of CNS β-amyloid in Alzheimer's disease. Science 330, 1774 (2010).
Patel, N.S. et al. Alzheimer's beta-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. J. Neurochem. 112, 66–76 (2010).
Yang, S.P. et al. Co-accumulation of vascular endothelial growth factor with beta-amyloid in the brain of patients with Alzheimer's disease. Neurobiol. Aging 25, 283–290 (2004).
Ruiz de Almodovar, C., Lambrechts, D., Mazzone, M. & Carmeliet, P. Role and therapeutic potential of VEGF in the nervous system. Physiol. Rev. 89, 607–648 (2009).
Wu, Z. et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat. Med. 11, 959–965 (2005).
Girouard, H. & Iadecola, C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 100, 328–335 (2006).
Chrissobolis, S., Miller, A.A., Drummond, G.R., Kemp-Harper, B.K. & Sobey, C.G. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front. Biosci. 16, 1733–1745 (2011).
Park, L. et al. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc. Natl. Acad. Sci. USA 108, 5063–5068 (2011).
Stewart, C.R. et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 (2010).
Xu, J. et al. Amyloid beta peptide-induced cerebral endothelial cell death involves mitochondrial dysfunction and caspase activation. J. Cereb. Blood Flow Metab. 21, 702–710 (2001).
Carrano, A. et al. Amyloid beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid. Redox Signal. 15, 1167–1178 (2011).
Thomas, T., Thomas, G., McLendon, C., Sutton, T. & Mullan, M. β-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature 380, 168–171 (1996).
Chow, N. et al. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer's phenotype. Proc. Natl. Acad. Sci. USA 104, 823–828 (2007).
Bell, R.D. et al. SRF and myocardin regulate LRP-mediated amyloid-β clearance in brain vascular cells. Nat. Cell Biol. 11, 143–153 (2009).
Hamilton, N.B., Attwell, D. & Hall, C.N. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front. Neuroenergetics 2, 5 (2010).
Peppiatt, C.M., Howarth, C., Mobbs, P. & Attwell, D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700–704 (2006).
Bell, R.D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
de la Torre, J.C. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 3, 184–190 (2004).
Greenberg, S.M., Gurol, M.E., Rosand, J. & Smith, E.E. Amyloid angiopathy-related vascular cognitive impairment. Stroke 35, 2616–2619 (2004).
Quaegebeur, A. & Carmeliet, P. Oxygen sensing: a common crossroad in cancer and neurodegeneration. Curr. Top. Microbiol. Immunol. 345, 71–103 (2010).
Liu, Y., Liu, F., Iqbal, K., Grundke-Iqbal, I. & Gong, C.X. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 582, 359–364 (2008).
Soucek, T., Cumming, R., Dargusch, R., Maher, P. & Schubert, D. The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid beta peptide. Neuron 39, 43–56 (2003).
Yemisci, M. et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 15, 1031–1037 (2009).
Grammas, P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer's disease. J. Neuroinflammation 8, 26 (2011).
Merlini, M., Meyer, E.P., Ulmann-Schuler, A. & Nitsch, R.M. Vascular β-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAβ mice. Acta Neuropathol. 122, 293–311 (2011).
Li, X., Lu, F., Wang, J.Z. & Gong, C.X. Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur. J. Neurosci. 23, 2078–2086 (2006).
Cunnane, S. et al. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition 27, 3–20 (2011).
Leen, W.G. et al. Glucose transporter-1 deficiency syndrome: the expanding clinical and genetic spectrum of a treatable disorder. Brain 133, 655–670 (2010).
Pasinelli, P. & Brown, R.H. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 7, 710–723 (2006).
Sutedja, N.A. et al. Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 82, 638–642 (2011).
Rule, R.R., Schuff, N., Miller, R.G. & Weiner, M.W. Gray matter perfusion correlates with disease severity in ALS. Neurology 74, 821–827 (2010).
Kew, J.J. et al. Cortical function in amyotrophic lateral sclerosis. A positron emission tomography study. Brain 116, 655–680 (1993).
Oosthuyse, B. et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 28, 131–138 (2001).
Zhong, Z. et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 11, 420–422 (2008).
Nicaise, C. et al. Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res. 1301, 152–162 (2009).
Henkel, J.S., Beers, D.R., Wen, S., Bowser, R. & Appel, S.H. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology 72, 1614–1616 (2009).
Garbuzova-Davis, S. et al. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS ONE 2, e1205 (2007).
Candelario-Jalil, E. et al. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke 42, 1345–1350 (2011).
Garbuzova-Davis, S. et al. Amyotrophic lateral sclerosis: A neurovascular disease. Brain Res. 1398, 113–125 (2011).
Derejko, M. et al. Regional cerebral blood flow in Parkinson's disease as an indicator of cognitive impairment. Nucl. Med. Commun. 27, 945–951 (2006).
Harris, G.J. et al. Reduced basal ganglia blood flow and volume in pre-symptomatic, gene-tested persons at-risk for Huntington's disease. Brain 122, 1667–1678 (1999).
Storkebaum, E. et al. Impaired autonomic regulation of resistance arteries in mice with low vascular endothelial growth factor or upon vascular endothelial growth factor trap delivery. Circulation 122, 273–281 (2010).
Storkebaum, E. et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat. Neurosci. 8, 85–92 (2005).
Liu, J. et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 (2009).
Lu, L. et al. Amyotrophic lateral sclerosis-linked mutant SOD1 sequesters Hu antigen R (HuR) and TIA-1-related protein (TIAR): implications for impaired post-transcriptional regulation of vascular endothelial growth factor. J. Biol. Chem. 284, 33989–33998 (2009).
Lambrechts, D. et al. Meta-analysis of vascular endothelial growth factor variations in amyotrophic lateral sclerosis: increased susceptibility in male carriers of the -2578AA genotype. J. Med. Genet. 46, 840–846 (2009).
Poesen, K. et al. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. J. Neurosci. 28, 10451–10459 (2008).
Greenway, M.J. et al. ANG mutations segregate with familial and 'sporadic' amyotrophic lateral sclerosis. Nat. Genet. 38, 411–413 (2006).
van Blitterswijk, M. & Landers, J.E. RNA processing pathways in amyotrophic lateral sclerosis. Neurogenetics 11, 275–290 (2010).
Tesseur, I. & Wyss-Coray, T. A role for TGF-beta signaling in neurodegeneration: evidence from genetically engineered models. Curr. Alzheimer Res. 3, 505–513 (2006).
Azzouz, M. et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 429, 413–417 (2004).
Dodge, J.C. et al. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol. Ther. 18, 2075–2084 (2010).
Wang, Y. et al. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J. Neurosci. 27, 304–307 (2007).
Xiong, N. et al. VEGF-expressing human umbilical cord mesenchymal stem cells, an improved therapy strategy for Parkinson's disease. Gene Ther. 18, 394–402 (2011).
Emerich, D.F., Mooney, D.J., Storrie, H., Babu, R.S. & Kordower, J.H. Injectable hydrogels providing sustained delivery of vascular endothelial growth factor are neuroprotective in a rat model of Huntington's disease. Neurotox. Res. 17, 66–74 (2010).
Spuch, C. et al. The effect of encapsulated VEGF-secreting cells on brain amyloid load and behavioral impairment in a mouse model of Alzheimer's disease. Biomaterials 31, 5608–5618 (2010).
Kieran, D. et al. Control of motoneuron survival by angiogenin. J. Neurosci. 28, 14056–14061 (2008).
Deane, R. et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 9, 907–913 (2003).
Town, T. et al. Blocking TGF-β–Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 14, 681–687 (2008).
Whitehead, K.J. et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat. Med. 15, 177–184 (2009).
Bose, P., Holter, J.L. & Selby, G.B. Bevacizumab in hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 360, 2143–2144 (2009).
Oosting, S., Nagengast, W., deVries, E., Retornaz, F. & Duvoux, C. More on bevacizumab in hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 361, 931; author reply 931–932 (2009).
Lebrin, F. et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat. Med. 16, 420–428 (2010).
Sagare, A. et al. Clearance of amyloid-β by circulating lipoprotein receptors. Nat. Med. 13, 1029–1031 (2007).
Faurobert, E. & Albiges-Rizo, C. Recent insights into cerebral cavernous malformations: a complex jigsaw puzzle under construction. FEBS J. 277, 1084–1096 (2010).
He, Y. et al. Stabilization of VEGFR2 signaling by cerebral cavernous malformation 3 is critical for vascular development. Sci. Signal. 3, ra26 (2010).
Kalaria, R.N. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr. Rev. 68 (suppl. 2) S74–S87 (2010).
Acknowledgements
We thank L. Notebaert, A. Truyens and B. Sass for help with the figures. E.S. is a fellow of FWO-Flanders, funded by the state North Rhine Westphalia and supported by a grant from the Frick Foundation for ALS Research. A.Q. is a fellow of the Fund for Scientific Research (FWO), Flanders. M.V. is supported by grants from the Interuniversity Attraction Poles initiated by the Belgian Science Policy, Network 5/25, the F.R.S.-FNRS (Fonds de la Recherche Scientifique), and the ARC (Actions de Recherche Concertées-Communauté française de Belgique). P.C. is supported by Long-term structural Methusalem funding by the Flemish Government, the Fund for Scientific Research–Flemish Government (FWO) (G.0677.09, G.0676.09), the Belgian Science Policy (IUAP-P6/30), the Association Française contre les Myopathies (AFM), Geneeskundige stichting Koningin Elisabeth and Motor Neurone Disease Association grant 70/130.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Storkebaum, E., Quaegebeur, A., Vikkula, M. et al. Cerebrovascular disorders: molecular insights and therapeutic opportunities. Nat Neurosci 14, 1390–1397 (2011). https://doi.org/10.1038/nn.2947
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2947
This article is cited by
-
Oxygen Sensing and Signaling in Alzheimer’s Disease: A Breathtaking Story!
Cellular and Molecular Neurobiology (2022)
-
Endothelial cell clonal expansion in the development of cerebral cavernous malformations
Nature Communications (2019)
-
Engineered 3D vascular and neuronal networks in a microfluidic platform
Scientific Reports (2018)
-
Quantitative assessment of angiogenesis, perfused blood vessels and endothelial tip cells in the postnatal mouse brain
Nature Protocols (2015)
-
Microvascular Lesions by Estrogen-Induced ID3: Its Implications in Cerebral and Cardiorenal Vascular Disease
Journal of Molecular Neuroscience (2015)