Abstract
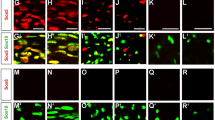
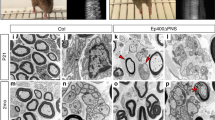
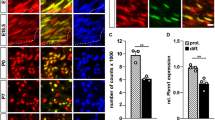
Histone deacetylases (HDACs) are major epigenetic regulators. We show that HDAC1 and HDAC2 functions are critical for myelination of the peripheral nervous system. Using mouse genetics, we have ablated Hdac1 and Hdac2 specifically in Schwann cells, resulting in massive Schwann cell loss and virtual absence of myelin in mutant sciatic nerves. Expression of Sox10 and Krox20, the main transcriptional regulators of Schwann cell myelination, was greatly reduced. We demonstrate that in Schwann cells, HDAC1 and HDAC2 exert specific primary functions: HDAC2 activates the transcriptional program of myelination in synergy with Sox10, whereas HDAC1 controls Schwann cell survival by regulating the levels of active β-catenin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Kadosh, D. & Struhl, K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell 89, 365–371 (1997).
Rundlett, S.E., Carmen, A.A., Suka, N., Turner, B.M. & Grunstein, M. Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature 392, 831–835 (1998).
Taunton, J., Hassig, C.A. & Schreiber, S.L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272, 408–411 (1996).
Zupkovitz, G. et al. Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol. Cell. Biol. 26, 7913–7928 (2006).
Wang, Z. et al. Genome-wide mapping of HATs and HDACs reveals distinct function in active and inactive genes. Cell 138, 1019–1031 (2009).
Glozak, M.A., Sengupta, N., Zhang, X. & Seto, E. Acetylation and deacetylation of non-histone proteins. Gene 363, 15–23 (2005).
Kazantsev, A.G. & Thompson, L.M. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat. Rev. Drug Discov. 7, 854–868 (2008).
Chuang, D.M., Leng, Y., Marinova, Z., Kim, H.J. & Chiu, C.T. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 32, 591–601 (2009).
Shen, S., Li, J. & Casaccia-Bonnefil, P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J. Cell Biol. 169, 577–589 (2005).
Shen, S. et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 11, 1024–1034 (2008).
Ye, F. et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the β-catenin–TCF interaction. Nat. Neurosci. 12, 829–838 (2009).
Bunge, M.B. Novel combination strategies to repair the injured mammalian spinal cord. J. Spinal Cord Med. 31, 262–269 (2008).
Ferner, R.E. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 6, 340–351 (2007).
Suter, U. & Scherer, S.S. Disease mechanisms in inherited neuropathies. Nat. Rev. Neurosci. 4, 714–726 (2003).
Yamaguchi, T. et al. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev. 24, 455–469 (2010).
Jaegle, M. et al. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 17, 1380–1391 (2003).
Joseph, N.M. et al. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 131, 5599–5612 (2004).
Montgomery, R.L. et al. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 21, 1790–1802 (2007).
Jessen, K.R. & Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 6, 671–682 (2005).
Parmantier, E. et al. Schwann cell–derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 23, 713–724 (1999).
Svaren, J. & Meijer, D. The molecular machinery of myelin gene transcription in Schwann cells. Glia 56, 1541–1551 (2008).
Zorick, T.S., Syroid, D.E., Brown, A., Gridley, T. & Lemke, G. Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development 126, 1397–1406 (1999).
Ryu, E.J. et al. Misexpression of Pou3f1 results in peripheral nerve hypomyelination and axonal loss. J. Neurosci. 27, 11552–11559 (2007).
Jessen, K.R. & Mirsky, R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia 56, 1552–1565 (2008).
Ghislain, J. & Charnay, P. Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 7, 52–58 (2006).
Zhang, J., Xu, F., Hashimshony, T., Keshet, I. & Cedar, H. Establishment of transcriptional competence in early and late S phase. Nature 420, 198–202 (2002).
Werner, T., Hammer, A., Wahlbuhl, M., Bösl, M.R. & Wegner, M. Multiple conserved regulatory elements with overlapping functions determine Sox10 expression in mouse embryogenesis. Nucleic Acids Res. 35, 6526–6538 (2007).
LeBlanc, S.E., Jang, S.-W., Ward, R.M., Wrabetz, L. & Svaren, J. Direct regulation of myelin protein zero expression by the Egr2 transactivator. J. Biol. Chem. 281, 5453–5460 (2006).
Bordonaro, M., Lazarova, D.L. & Sartorelli, A.C. The activation of beta-catenin by Wnt signaling mediates the effects of histone deacetylase inhibitors. Exp. Cell Res. 313, 1652–1666 (2007).
Finzsch, M. et al. Sox10 is not only required for Schwann cell specification, but also for maintenance of cell identity and progression beyond the immature Schwann cell stage. J. Cell Biol. 189, 701–712 (2010).
Topilko, P. et al. Krox-20 controls myelination in the peripheral nervous system. Nature 371, 796–799 (1994).
Clevers, H. & van de Wetering, M. TCF/LEF factor earn their wings. Trends Genet. 13, 485–489 (1997).
Dutton, J.R. et al. An evolutionarily conserved intronic region controls the spatiotemporal expression of the transcription factor Sox10. BMC Dev. Biol. 8, 105–124 (2008).
Chen, Y. et al. HDAC-mediated deacetylation of NF-κB is critical for Schwann cell myelination. Nat. Neurosci. advance online publication, doi:10.1038/nn.2780 (20 March 2011).
Lindeboom, F. et al. A tissue-specific knockout reveals that Gata1 is not essential for Sertoli cell function in the mouse. Nucleic Acids Res. 31, 5405–5412 (2003).
Pereira, J.A. et al. Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J. Cell Biol. 185, 147–161 (2009).
Jacob, C., Grabner, H., Atanasoski, S. & Suter, U. Expression and localization of Ski determine cell type-specific TGFβ signaling effects on the cell cycle. J. Cell Biol. 182, 519–530 (2008).
Welm, B.E., Dijkgraaf, G.J., Bledau, A.S., Welm, A.L. & Werb, Z. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell 2, 90–102 (2008).
Barth, A.I., Stewart, D.B. & Nelson, W.J. T cell factor-activated transcription is not sufficient to induce anchorage-independent growth of epithelial cells expressing mutant β-catenin. Proc. Natl. Acad. Sci. USA 96, 4947–4952 (1999).
Ghazvini, M. et al. A cell type-specific allele of the POU gene Oct-6 reveals Schwann cell autonomous function in nerve development and regeneration. EMBO J. 21, 4612–4620 (2002).
Pereira, J.A. et al. Dicer in Schwann cells is required for myelination and axonal integrity. J. Neurosci. 30, 6763–6775 (2010).
Antonellis, A. et al. Identification of neural crest and glial enhancers at the mouse Sox10 locus through transgenesis in zebrafish. PLoS Genet. 4, e1000174 (2008).
Reiprich, S., Kriesch, J., Schreiner, S. & Wegner, M. Activation of Krox20 gene expression by Sox10 in myelinating Schwann cells. J. Neurochem. 112, 744–754 (2010).
Kao, S.-C. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science 323, 651–654 (2009).
Acknowledgements
We thank M. Wegner (Universtät Erlangen-Nürnberg), E. Seto (Moffitt Cancer Center), S. Schreiber (Harvard University), Z. Werb and B. Welm (University of Utah), J. Crabtree (Stanford University), T. Reya (Duke University Medical Center), W.J. Pavan (US National Institutes of Health), A. McCallion and M. Prasad (Johns Hopkins University School of Medicine) and D.R. Colman (McGill University) for providing constructs and antibodies, T. Meier, W. Seufert and N. Mantei for critically reading the manuscript and the Functional Genomic Center Zurich for gene array analyses. These studies were supported by two Swiss National Science Foundation (SNSF) grants, a Marie-Heim Vögtlin subsidy (PMPDP3_122738/1) to C. Jacob, and another SNSF grant to U. Suter, and by a grant from the Swiss National Center for Competence in Research (NCCR) Neural Plasticity and Repair to U. Suter. The work in the laboratory of P. Matthias was supported by the Novartis Research Foundation.
Author information
Authors and Affiliations
Contributions
C.J. designed the study, analyzed the data and wrote the manuscript; C.J., C.N.C., J.A.P., C.S., A.B, P.L., M.O. and N.T. performed and analyzed the experiments; D.M., T.Y. and P.M. generated and provided crucial mouse lines and support; U.S. conceived and supervised the study and co-wrote the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 14308 kb)
Rights and permissions
About this article
Cite this article
Jacob, C., Christen, C., Pereira, J. et al. HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat Neurosci 14, 429–436 (2011). https://doi.org/10.1038/nn.2762
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2762
This article is cited by
-
Evaluation of photobiomodulation therapy (117 and 90s) on pain, regeneration, and epigenetic factors (HDAC 2, DNMT3a) expression following spinal cord injury in a rat model
Photochemical & Photobiological Sciences (2023)
-
Transcriptome Analysis of Schwann Cells at Various Stages of Myelination Implicates Chromatin Regulator Sin3A in Control of Myelination Identity
Neuroscience Bulletin (2022)
-
Neuronal lineages derived from the nerve-associated Schwann cell precursors
Cellular and Molecular Life Sciences (2021)
-
Myelin Biology
Neurotherapeutics (2021)
-
Peripheral Nerve Development and the Pathogenesis of Peripheral Neuropathy: the Sorting Point
Neurotherapeutics (2021)