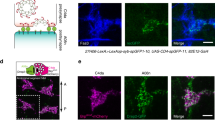
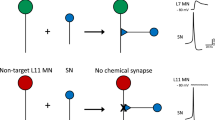
Abstract
Adrenergic signaling has important roles in synaptic plasticity and metaplasticity. However, the underlying mechanisms of these functions remain poorly understood. We investigated the role of octopamine, the invertebrate counterpart of adrenaline and noradrenaline, in synaptic and behavioral plasticity in Drosophila. We found that an increase in locomotor speed induced by food deprivation was accompanied by an activity- and octopamine-dependent extension of octopaminergic arbors and that the formation and maintenance of these arbors required electrical activity. Growth of octopaminergic arbors was controlled by a cAMP- and CREB-dependent positive-feedback mechanism that required Octβ2R octopamine autoreceptors. Notably, this autoregulation was necessary for the locomotor response. In addition, octopamine neurons regulated the expansion of excitatory glutamatergic neuromuscular arbors through Octβ2Rs on glutamatergic motor neurons. Our results provide a mechanism for global regulation of excitatory synapses, presumably to maintain synaptic and behavioral plasticity in a dynamic range.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
16 January 2011
In the version of this article initially published online, an error was made on page 2, left column, third paragraph, sixth line. The genotype 'tbhnM19' should read 'tbhnM18'. In the Online Methods, left column, first paragraph, fifth line, the genotype 'tbhnM189' should read 'tbhnM18 (ref. 9)'. Finally, in the Online Methods, right column, seventh paragraph, fourth line, the abbreviation 'Drc' should read 'Dcr'. These errors have been corrected for the print, PDF and HTML versions of this article.
References
Murchison, C.F. et al. A distinct role for norepinephrine in memory retrieval. Cell 117, 131–143 (2004).
Hu, H. et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 131, 160–173 (2007).
Kuzmiski, J.B., Pittman, Q.J. & Bains, J.S. Metaplasticity of hypothalamic synapses following in vivo challenge. Neuron 62, 839–849 (2009).
Balfanz, S., Strunker, T., Frings, S. & Baumann, A. A family of octopamine [corrected] receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J. Neurochem. 93, 440–451 (2005).
Hammer, M. & Menzel, R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 5, 146–156 (1998).
Schroll, C. et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16, 1741–1747 (2006).
Schwaerzel, M. et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10495–10502 (2003).
Zhou, C. & Rao, Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat. Neurosci. 11, 1059–1067 (2008).
Monastirioti, M., Linn, C.E. Jr. & White, K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 16, 3900–3911 (1996).
Suo, S., Kimura, Y. & Van Tol, H.H. Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine-Gq signaling in Caenorhabditis elegans. J. Neurosci. 26, 10082–10090 (2006).
Crocker, A., Shahidullah, M., Levitan, I.B. & Sehgal, A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 65, 670–681 (2010).
Breen, C.A. & Atwood, H.L. Octopamine—a neurohormone with presynaptic activity-dependent effects at crayfish neuromuscular junctions. Nature 303, 716–718 (1983).
Monastirioti, M. et al. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J. Comp. Neurol. 356, 275–287 (1995).
Schuster, C.M., Davis, G.W., Fetter, R.D. & Goodman, C.S. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron 17, 655–667 (1996).
Marqués, G. & Zhang, B. Retrograde signaling that regulates synaptic development and function at the Drosophila neuromuscular junction. Int. Rev. Neurobiol. 75, 267–285 (2006).
Korkut, C. & Budnik, V. WNTs tune up the neuromuscular junction. Nat. Rev. Neurosci. 10, 627–634 (2009).
Steinert, J.R. et al. Experience-dependent formation and recruitment of large vesicles from reserve pool. Neuron 50, 723–733 (2006).
Sigrist, S.J. et al. Experience-dependent strengthening of Drosophila neuromuscular junctions. J. Neurosci. 23, 6546–6556 (2003).
Budnik, V., Zhong, Y. & Wu, C.F. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J. Neurosci. 10, 3754–3768 (1990).
Ataman, B. et al. Rapid activity-dependent modifications in synaptic structure and function require bidirectional wnt signaling. Neuron 57, 705–718 (2008).
Saraswati, S., Fox, L.E., Soll, D.R. & Wu, C.F. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J. Neurobiol. 58, 425–441 (2004).
Davenport, A.P. & Evans, P.D. Changes in haemolymph octopamine levels associated with food deprivation in the locust, Schistocerca gregaria. Physiol. Entomol. 9, 269–274 (1984).
Hardie, S.L., Zhang, J.X. & Hirsh, J. Trace amines differentially regulate adult locomotor activity, cocaine sensitivity, and female fertility in Drosophila melanogaster. Dev. Neurobiol. 67, 1396–1405 (2007).
Kittel, R.J. et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science 312, 1051–1054 (2006).
Rasse, T.M. et al. Glutamate receptor dynamics organizing synapse formation in vivo. Nat. Neurosci. 8, 898–905 (2005).
Fouquet, W. et al. Maturation of active zone assembly by Drosophila Bruchpilot. J. Cell Biol. 186, 129–145 (2009).
Mosca, T.J., Carrillo, R.A., White, B.H. & Keshishian, H. Dissection of synaptic excitability phenotypes by using a dominant-negative Shaker K+ channel subunit. Proc. Natl. Acad. Sci. USA 102, 3477–3482 (2005).
Kitamoto, T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92 (2001).
White, B.H. et al. Targeted attenuation of electrical activity in Drosophila using a genetically modified K(+) channel. Neuron 31, 699–711 (2001).
Paradis, S., Sweeney, S.T. & Davis, G.W. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron 30, 737–749 (2001).
McGuire, S.E., Mao, Z. & Davis, R.L. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE 2004, pl6 (2004).
Han, K.A., Millar, N.S. & Davis, R.L. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J. Neurosci. 18, 3650–3658 (1998).
Zhong, Y., Budnik, V. & Wu, C.F. Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J. Neurosci. 12, 644–651 (1992).
Schröder-Lang, S. et al. Fast manipulation of cellular cAMP level by light in vivo. Nat. Methods 4, 39–42 (2007).
Benito, E. & Barco, A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 33, 230–240 (2010).
Perazzona, B., Isabel, G., Preat, T. & Davis, R.L. The role of cAMP response element-binding protein in Drosophila long-term memory. J. Neurosci. 24, 8823–8828 (2004).
Lee, H.G. et al. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev. Biol. 264, 179–190 (2003).
Schuster, C.M., Davis, G.W., Fetter, R.D. & Goodman, C.S. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17, 641–654 (1996).
O'Dell, T.J., Connor, S.A., Gelinas, J.N. & Nguyen, P.V. Viagra for your synapses: enhancement of hippocampal long-term potentiation by activation of beta-adrenergic receptors. Cell. Signal. 22, 728–736 (2010).
Roeder, T. Tyramine and octopamine: ruling behavior and metabolism. Annu. Rev. Entomol. 50, 447–477 (2005).
Howell, K.M. & Evans, P.D. The characterization of presynaptic octopamine receptors modulating octopamine release from an identified neurone in the locust. J. Exp. Biol. 201, 2053–2060 (1998).
Floresco, S.B. et al. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 6, 968–973 (2003).
Aamodt, S.M. & Constantine-Paton, M. The role of neural activity in synaptic development and its implications for adult brain function. Adv. Neurol. 79, 133–144 (1999).
Landgraf, M., Bossing, T., Technau, G.M. & Bate, M. The origin, location, and projections of the embryonic abdominal motorneurons of Drosophila. J. Neurosci. 17, 9642–9655 (1997).
Takizawa, E., Komatsu, A. & Tsujimura, H. Identification of common excitatory motoneurons in Drosophila melanogaster larvae. Zoolog. Sci. 24, 504–513 (2007).
Nagaya, Y., Kutsukake, M., Chigusa, S.I. & Komatsu, A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci. Lett. 329, 324–328 (2002).
Kutsukake, M., Komatsu, A., Yamamoto, D. & Ishiwa-Chigusa, S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster. Gene 245, 31–42 (2000).
Nishikawa, K. & Kidokoro, Y. Octopamine inhibits synaptic transmission at the larval neuromuscular junction in Drosophila melanogaster. Brain Res. 837, 67–74 (1999).
McNabb, S.L. et al. Disruption of a behavioral sequence by targeted death of peptidergic neurons in Drosophila. Neuron 19, 813–823 (1997).
Cheung, U.S., Shayan, A.J., Boulianne, G.L. & Atwood, H.L. Drosophila larval neuromuscular junction's responses to reduction of cAMP in the nervous system. J. Neurobiol. 40, 1–13 (1999).
Acknowledgements
We thank M. Yoshihara, S. Speese, Y. Fuentes-Medel and C. Korkut for comments on the manuscript, C. Brewer for assistance with data analysis and the UMass Amherst antibody facility for production of the TBH antibody. This work was supported by US National Institutes of Health grants R01 MH0700000 to V.B., MH09883 to S.W., MH081982 to S.W. and GM084491 to M.J.A. M.J.A. was also supported by the Bill & Melinda Gates Foundation.
Author information
Authors and Affiliations
Contributions
A.C.K. designed and performed most experiments and contributed to manuscript writing; J.A. contributed to tool development, electrophysiology, experimental design and manuscript writing; R.B. performed RT-PCR and some immunocytochemistry; S.W., S.D. and R.B. generated, characterized and validated PACα function; M.J.A. helped with the design of the TBH antibody; and V.B. directed the project and wrote the manuscript in collaboration with A.C.K. and J.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 894 kb)
Supplementary Movie 1
Dynamics of natural synaptopods at type-II arbors. (MOV 18 kb)
Supplementary Movie 2
Synaptopods develop ball-shaped varicosities. (MOV 252 kb)
Supplementary Movie 3
Secondary synaptopod formation on a varicosity. (MOV 109 kb)
Supplementary Movie 4
Induction of synaptopod formation upon acute increase in cAMP levels. (MOV 1384 kb)
Rights and permissions
About this article
Cite this article
Koon, A., Ashley, J., Barria, R. et al. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat Neurosci 14, 190–199 (2011). https://doi.org/10.1038/nn.2716
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2716
This article is cited by
-
A neural m6A pathway regulates behavioral aggregation in migratory locusts
Science China Life Sciences (2024)
-
The effects of methylphenidate and atomoxetine on Drosophila brain at single-cell resolution and potential drug repurposing for ADHD treatment
Molecular Psychiatry (2023)
-
Single cell RNA-seq analysis reveals temporally-regulated and quiescence-regulated gene expression in Drosophila larval neuroblasts
Neural Development (2022)
-
Maintenance of quiescent oocytes by noradrenergic signals
Nature Communications (2021)
-
Aggressive Behaviour of Drosophila suzukii in Relation to Environmental and Social Factors
Scientific Reports (2020)