Abstract
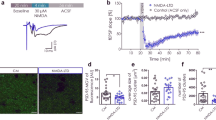
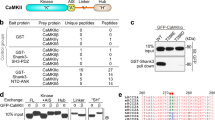
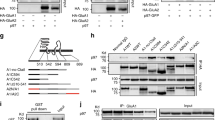
Homeostatic plasticity is crucial for maintaining neuronal output by counteracting unrestrained changes in synaptic strength. Chronic elevation of synaptic activity by bicuculline reduces the amplitude of miniature excitatory postsynaptic currents (mEPSCs), but the underlying mechanisms of this effect remain unclear. We found that activation of EphA4 resulted in a decrease in synaptic and surface GluR1 and attenuated mEPSC amplitude through a degradation pathway that requires the ubiquitin proteasome system (UPS). Elevated synaptic activity resulted in increased tyrosine phosphorylation of EphA4, which associated with the ubiquitin ligase anaphase-promoting complex (APC) and its activator Cdh1 in neurons in a ligand-dependent manner. APCCdh1 interacted with and targeted GluR1 for proteasomal degradation in vitro, whereas depletion of Cdh1 in neurons abolished the EphA4-dependent downregulation of GluR1. Knockdown of EphA4 or Cdh1 prevented the reduction in mEPSC amplitude in neurons that was a result of chronic elevated activity. Our results define a mechanism by which EphA4 regulates homeostatic plasticity through an APCCdh1-dependent degradation pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Turrigiano, G.G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135, 422–435 (2008).
Turrigiano, G.G., Leslie, K.R., Desai, N.S., Rutherford, L.C. & Nelson, S.B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896 (1998).
Shepherd, J.D. et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52, 475–484 (2006).
Stellwagen, D. & Malenka, R.C. Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059 (2006).
Bingol, B. & Schuman, E.M. Synaptic protein degradation by the ubiquitin proteasome system. Curr. Opin. Neurobiol. 15, 536–541 (2005).
Yi, J.J. & Ehlers, M.D. Ubiquitin and protein turnover in synapse function. Neuron 47, 629–632 (2005).
Haglund, K., Di Fiore, P.P. & Dikic, I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem. Sci. 28, 598–603 (2003).
Ciechanover, A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Cell Death Differ. 12, 1178–1190 (2005).
Zhao, Y., Hegde, A.N. & Martin, K.C. The ubiquitin proteasome system functions as an inhibitory constraint on synaptic strengthening. Curr. Biol. 13, 887–898 (2003).
Patrick, G.N., Bingol, B., Weld, H.A. & Schuman, E.M. Ubiquitin-mediated proteasome activity is required for agonist-induced endocytosis of GluRs. Curr. Biol. 13, 2073–2081 (2003).
Jordan, B.A. et al. Identification and verification of novel rodent postsynaptic density proteins. Mol. Cell. Proteomics 3, 857–871 (2004).
Li, K.W. et al. Proteomics analysis of rat brain postsynaptic density. Implications of the diverse protein functional groups for the integration of synaptic physiology. J. Biol. Chem. 279, 987–1002 (2004).
Ehlers, M.D. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 6, 231–242 (2003).
Burbea, M., Dreier, L., Dittman, J.S., Grunwald, M.E. & Kaplan, J.M. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron 35, 107–120 (2002).
Juo, P. & Kaplan, J.M. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr. Biol. 14, 2057–2062 (2004).
van Roessel, P., Elliott, D.A., Robinson, I.M., Prokop, A. & Brand, A.H. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell 119, 707–718 (2004).
Colledge, M. et al. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron 40, 595–607 (2003).
Zhang, D. et al. Na,K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J. Neurosci. 29, 4498–4511 (2009).
Pasquale, E.B. Eph-Ephrin bidirectional signaling in physiology and disease. Cell 133, 38–52 (2008).
Klein, R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat. Neurosci. 12, 15–20 (2009).
Deininger, K. et al. The Rab5 guanylate exchange factor Rin1 regulates endocytosis of the EphA4 receptor in mature excitatory neurons. Proc. Natl. Acad. Sci. USA 105, 12539–12544 (2008).
Murai, K.K., Nguyen, L.N., Irie, F., Yamaguchi, Y. & Pasquale, E.B. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat. Neurosci. 6, 153–160 (2003).
Fu, W.Y. et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat. Neurosci. 10, 67–76 (2007).
Penzes, P. et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron 37, 263–274 (2003).
Henkemeyer, M., Itkis, O.S., Ngo, M., Hickmott, P.W. & Ethell, I.M. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J. Cell Biol. 163, 1313–1326 (2003).
Dalva, M.B. et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 103, 945–956 (2000).
Takasu, M.A., Dalva, M.B., Zigmond, R.E. & Greenberg, M.E. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science 295, 491–495 (2002).
Wierenga, C.J., Ibata, K. & Turrigiano, G.G. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J. Neurosci. 25, 2895–2905 (2005).
O'Brien, R.J. et al. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron 21, 1067–1078 (1998).
Malinow, R. AMPA receptor trafficking and long-term potentiation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358, 707–714 (2003).
Bredt, D.S. & Nicoll, R.A. AMPA receptor trafficking at excitatory synapses. Neuron 40, 361–379 (2003).
Cheng, K. et al. Pctaire1 interacts with p35 and is a novel substrate for Cdk5/p35. J. Biol. Chem. 277, 31988–31993 (2002).
Castro, A., Bernis, C., Vigneron, S., Labbe, J.C. & Lorca, T. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene 24, 314–325 (2005).
Konishi, Y., Stegmuller, J., Matsuda, T., Bonni, S. & Bonni, A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science 303, 1026–1030 (2004).
Gieffers, C., Peters, B.H., Kramer, E.R., Dotti, C.G. & Peters, J.M. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc. Natl. Acad. Sci. USA 96, 11317–11322 (1999).
Kraft, C., Vodermaier, H.C., Maurer-Stroh, S., Eisenhaber, F. & Peters, J.M. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol. Cell 18, 543–553 (2005).
Kato, A., Rouach, N., Nicoll, R.A. & Bredt, D.S. Activity-dependent NMDA receptor degradation mediated by retrotranslocation and ubiquitination. Proc. Natl. Acad. Sci. USA 102, 5600–5605 (2005).
Lim, K.L. et al. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J. Neurosci. 25, 2002–2009 (2005).
Matyskiela, M.E., Rodrigo-Brenni, M.C. & Morgan, D.O. Mechanisms of ubiquitin transfer by the anaphase-promoting complex. J. Biol. 8, 92 (2009).
Pozo, K. & Goda, Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66, 337–351 (2010).
Lissin, D.V. et al. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc. Natl. Acad. Sci. USA 95, 7097–7102 (1998).
O'Brien, R.J., Lau, L.F. & Huganir, R.L. Molecular mechanisms of glutamate receptor clustering at excitatory synapses. Curr. Opin. Neurobiol. 8, 364–369 (1998).
Seeburg, D.P., Feliu-Mojer, M., Gaiottino, J., Pak, D.T. & Sheng, M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron 58, 571–583 (2008).
Ang, X.L., Seeburg, D.P., Sheng, M. & Harper, J.W. Regulation of postsynaptic RapGAP SPAR by Polo-like kinase 2 and the SCFbeta-TRCP ubiquitin ligase in hippocampal neurons. J. Biol. Chem. 283, 29424–29432 (2008).
Helton, T.D., Otsuka, T., Lee, M.C., Mu, Y. & Ehlers, M.D. Pruning and loss of excitatory synapses by the parkin ubiquitin ligase. Proc. Natl. Acad. Sci. USA 105, 19492–19497 (2008).
Murthy, V.N., Schikorski, T., Stevens, C.F. & Zhu, Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron 32, 673–682 (2001).
Burrone, J., O'Byrne, M. & Murthy, V.N. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature 420, 414–418 (2002).
Listovsky, T. et al. Mammalian Cdh1/Fzr mediates its own degradation. EMBO J. 23, 1619–1626 (2004).
Sala, C. et al. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron 31, 115–130 (2001).
Sala, C. et al. Inhibition of dendritic spine morphogenesis and synaptic transmission by activity-inducible protein Homer1a. J. Neurosci. 23, 6327–6337 (2003).
Acknowledgements
We thank P. Bartlett for EphA4−/− mice, R. Huganir for GluR1 antibodies and the GluR1 4KR mutant expression construct, R. Malinow for the GluR1-GFP construct, J. Chamberlain for the mouse muscle cDNA library, L. Shi, W. Chau, K. Kong, X.-N. Li, W. Fang, E. Cheung, B. Butt and C. Kwong for technical assistance, and members of the Ip laboratory for discussions. This study was supported in part by the Research Grants Council of Hong Kong SAR (HKUST, 6444/06M, 661007, 661109, 1/06C and 6/CRF/08), the Area of Excellence Scheme of the University Grants Committee (AoE/B-15/01) and the Hong Kong Jockey Club. N.Y. Ip and K.-O. Lai were recipients of the Croucher Foundation Senior Research Fellowship and Croucher Foundation Fellowship, respectively.
Author information
Authors and Affiliations
Contributions
N.Y.I. supervised the project. A.K.Y.F., K.-W.H., W.-Y.F., K.-O.L. and N.Y.I. designed the experiments. K.-W.H., W.-Y.F., Y.C. and K.-O.L. conducted the majority of experiments. A.K.Y.F., K.-W.H., W.-Y.F., Y.C., K.-O.L. and N.Y.I. did the data analyses. J.X. designed and did the data analyses on the electrophysiology experiment and C.S. performed electrophysiology experiment. A.K.Y.F., K.-O.L. and N.Y.I. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 1398 kb)
Rights and permissions
About this article
Cite this article
Fu, A., Hung, KW., Fu, WY. et al. APCCdh1 mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat Neurosci 14, 181–189 (2011). https://doi.org/10.1038/nn.2715
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2715
This article is cited by
-
Emergence of consciousness from anesthesia through ubiquitin degradation of KCC2 in the ventral posteromedial nucleus of the thalamus
Nature Neuroscience (2023)
-
Early alterations in the MCH system link aberrant neuronal activity and sleep disturbances in a mouse model of Alzheimer’s disease
Nature Neuroscience (2023)
-
Remodeling without destruction: non-proteolytic ubiquitin chains in neural function and brain disorders
Molecular Psychiatry (2021)
-
UPF2 leads to degradation of dendritically targeted mRNAs to regulate synaptic plasticity and cognitive function
Molecular Psychiatry (2020)
-
EphA4 loss improves social memory performance and alters dendritic spine morphology without changes in amyloid pathology in a mouse model of Alzheimer’s disease
Alzheimer's Research & Therapy (2019)