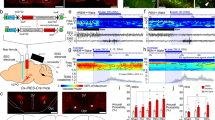
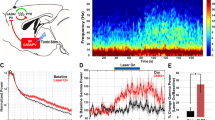
Abstract
Neural activity in the noradrenergic locus coeruleus correlates with periods of wakefulness and arousal. However, it is unclear whether tonic or phasic activity in these neurons is necessary or sufficient to induce transitions between behavioral states and to promote long-term arousal. Using optogenetic tools in mice, we found that there is a frequency-dependent, causal relationship among locus coeruleus firing, cortical activity, sleep-to-wake transitions and general locomotor arousal. We also found that sustained, high-frequency stimulation of the locus coeruleus at frequencies of 5 Hz and above caused reversible behavioral arrests. These results suggest that the locus coeruleus is finely tuned to regulate organismal arousal and that bursts of noradrenergic overexcitation cause behavioral attacks that resemble those seen in people with neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aston-Jones, G. & Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
Berridge, C.W. & Waterhouse, B.D. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 42, 33–84 (2003).
Foote, S.L., Bloom, F.E. & Aston-Jones, G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol. Rev. 63, 844–914 (1983).
Saper, C.B., Scammell, T.E. & Lu, J. Hypothalamic regulation of sleep and circadian rhyhms. Nature 437, 1257–1263 (2005).
Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 10, 211–223 (2009).
Aston-Jones, G. & Bloom, F.E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1, 876–886 (1981).
Aston-Jones, G. & Bloom, F.E. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J. Neurosci. 1, 887–900 (1981).
Hobson, J.A., McCarley, R.W. & Wyzinski, P.W. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 189, 55–58 (1975).
Foote, S.L., Aston-Jones, G. & Bloom, F.E. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. USA 77, 3033–3037 (1980).
Jones, B.E., Harper, S.T. & Halaris, A.E. Effects of locus coeruleus lesions upon cerebral monoamine content, sleep-wakefulness states and the response to amphetamine in the cat. Brain Res. 124, 473–496 (1977).
Lidbrink, P. The effect of lesions of ascending noradrenaline pathways on sleep and waking in the rat. Brain Res. 74, 19–40 (1974).
Blanco-Centurion, C., Gerashchenko, D. & Shiromani, P.J. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J. Neurosci. 27, 14041–14048 (2007).
Hunsley, M.S. & Palmiter, R.D. Norepinephrine-deficient mice exhibit normal sleep-wake states but have shorter sleep latency after mild stress and low doses of amphetamine. Sleep 26, 521–526 (2003).
Berridge, C.W. & Espana, R.A. Synergistic sedative effects of noradrenergic alpha(1)- and beta-receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience 99, 495–505 (2000).
De Sarro, G.B., Ascioti, C., Froio, F., Libri, V. & Nistico, F. Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha 1- and alpha 2-adrenoceptors affect sleep and arousal mechanisms. Br. J. Pharmacol. 90, 675–685 (1987).
Flicker, C. & Geyer, M.A. The hippocampus as a possible site of action for increased locomotion during intracerebral infusions of norepinephrine. Behav. Neural Biol. 34, 421–426 (1982).
Segal, D.S. & Mandell, A.J. Behavioral activation of rats during intraventricular infusion of norepinephrine. Proc. Natl. Acad. Sci. USA 66, 289–293 (1970).
Berridge, C.W. & Foote, S.L. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J. Neurosci. 11, 3135–3145 (1991).
Gradinaru, V. et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci. 27, 14231–14238 (2007).
Zhang, F., Aravanis, A.M., Adamantidis, A., de Lecea, L. & Deisseroth, K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat. Rev. Neurosci. 8, 577–581 (2007).
Adamantidis, A., Zhang, F., Aravanis, A.M., Deisseroth, K. & de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424 (2007).
Carter, M.E., Adamantidis, A., Ohtsu, H., Deisseroth, K. & de Lecea, L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J. Neurosci. 29, 10939–10949 (2009).
Gradinaru, V., Thompson, K.R. & Deisseroth, K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 36, 129–139 (2008).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Sohal, V.S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009).
Tsai, H.C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Lindeberg, J. et al. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis 40, 67–73 (2004).
Paxinos, G. & Franklin, K. The Mouse Brain in Stereotaxic Coordinates. 2nd edn. (Academic, New York, 2001).
Shipley, M.T. et al. Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. J. Comp. Neurol. 365, 56–68 (1996).
Bourgin, P. et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J. Neurosci. 20, 7760–7765 (2000).
Valentino, R. et al. Corticotropin-releasing factor innervation of the locus coeruleus region: distribution of fibers and sources of input. Neuroscience 48, 689–705 (1992).
van Bockstaele, E.J. et al. Anatomic basis for differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biol. Psychiatry 46, 1352–1363 (1999).
Jodo, E., Chiang, C. & Aston-Jones, G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coreuleus neurons. Neuroscience 83, 63–79 (1998).
Luquet, S., Perez, F.A., Hnasko, T.S. & Palmiter, R.D. NPY/AgRP neurons are essential for feeding in adult mice, but can be ablated in neonates. Science 310, 683–685 (2005).
Wu, Q., Boyle, M.P. & Palmiter, R.D. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137, 1225–1234 (2009).
Szot, P. et al. A comprehensive analysis of the effect of DSP4 on the locus coeruleus noradrenergic system in the rat. Neuropharmacology 166, 279–291 (2010).
Parmentier, R. et al. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J. Neurosci. 22, 7695–7711 (2002).
McGinty, D.J. & Harper, R.M. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 101, 569–575 (1976).
Steriade, M. Acetycholine systems and rhythmic activities during the waking-sleep cycle. Prog. Brain Res. 145, 179–196 (2004).
Boucetta, S. & Jones, B.E. Activity profiles of cholinergic and intermingled GABAergic and putative glutamatergic neurons in the pontomesencelphalic tegmentum of urethane-anesthetized rats. J. Neurosci. 29, 4664–4674 (2009).
Hassani, O.K., Lee, M.G., Henny, P. & Jones, B.E. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J. Neurosci. 29, 11828–11840 (2009).
Arnsten, A.F. Stress signaling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10, 410–422 (2009).
Ramos, B.P. & Arnsten, A. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol. Ther. 113, 523–536 (2007).
Bouret, S. & Sara, S.J. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582 (2005).
Wu, M.F. et al. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J. Physiol. (Lond.) 554, 202–215 (2004).
Lai, Y.Y., Kodama, T. & Siegel, J.M. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J. Neurosci. 21, 7384–7391 (2001).
Kodama, T., Lai, Y.Y. & Siegel, J.M. Changes in inhibitory amino acid release linked to pontine-induced atonia: an in vivo microdialysis study. J. Neurosci. 23, 1548–1554 (2003).
Scammell, T.E. et al. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep 32, 111–116 (2009).
Wu, M.F. et al. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience 91, 1389–1399 (1999).
Acknowledgements
We thank members of the de Lecea laboratory for helpful advice and feedback, J. Shieh for assistance with confocal images, A. Hilgendorff for assistance with mouse cardiovascular measurements and S. Xie for access to custom-built SmartCages. M.E.C. received financial support from an NSF Graduate Research Fellowship and the US National Institutes of Health (NIH) National Research Service Award (F31MH83439). O.Y. is supported by a European Molecular Biology Organization long-term postdoctoral fellowship. S.C. is supported by the Excellent Young Researcher Overseas Visit Program (21-8162) of the Japan Society for the Promotion of Science. A.A. is supported by fellowships from the Fonds National de la Recherche Scientifique ('Charge de Recherche'), NIH (K99) and NARSAD. S.N. is supported by NIH grant R01MH072525. K.D. is supported by the National Science Foundation, National Institute of Mental Health, National Institute on Drug Abuse, and the McKnight, Coulter, Snyder, Albert Yu and Mary Bechmann, and Keck Foundations. L.d.L. is supported by the NIH (MH83702, MH87592, DA21880) and NARSAD.
Author information
Authors and Affiliations
Contributions
M.E.C. and L.d.L. designed the study and wrote the manuscript. M.E.C. performed or assisted with all experiments. O.Y. performed and analyzed electrophysiology experiments, S.C. performed HPLC analysis, and H.N. analyzed immunohistochemical co-expression data. A.A. and L.d.L. provided expertise on optogenetic and polysomnographic recording techniques, as well as substantial feedback on the manuscript. S.N., K.D. and L.d.L. provided equipment, reagents and critical feedback.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 8230 kb)
Supplementary Movie 1
Representative sleep-to-wake transition following photostimulation of locus coeruleus neurons during NREM sleep. Photostimulation condition was 10 ms pulses at 5 Hz for 5 s. (MOV 902 kb)
Supplementary Movie 2
Representative behavioral arrest following sustained, high-frequency photostimulation of locus coeruleus neurons with 10 ms pulses at 10 Hz. (MOV 3007 kb)
Rights and permissions
About this article
Cite this article
Carter, M., Yizhar, O., Chikahisa, S. et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 13, 1526–1533 (2010). https://doi.org/10.1038/nn.2682
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2682
This article is cited by
-
Electrophysiological correlates of attention in the locus coeruleus–prelimbic cortex circuit during the rodent continuous performance test
Neuropsychopharmacology (2024)
-
Sexual differences in locus coeruleus neurons and related behavior in C57BL/6J mice
Biology of Sex Differences (2023)
-
Neuro-orchestration of sleep and wakefulness
Nature Neuroscience (2023)
-
A common neuronal ensemble in nucleus accumbens regulates pain-like behaviour and sleep
Nature Communications (2023)
-
Adrenergic signalling to astrocytes in anterior cingulate cortex contributes to pain-related aversive memory in rats
Communications Biology (2023)