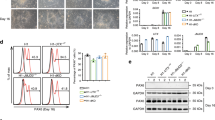
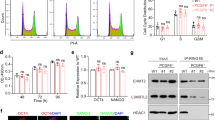
Abstract
Neural stem cells (NSCs) are uncommitted cells of the CNS defined by their multipotentiality and ability to self renew. We found these cells to not be present in substantial numbers in the CNS until after embryonic day (E) 10.5 in mouse and E5 in chick. This coincides with the induction of SOX9 in neural cells. Gain- and loss-of-function studies indicated that SOX9 was essential for multipotent NSC formation. Moreover, Sonic Hedgehog was able to stimulate precocious generation of NSCs by inducing Sox9 expression. SOX9 was also necessary for the maintenance of multipotent NSCs, as shown by in vivo fate mapping experiments in the adult subependymal zone and olfactory bulbs. In addition, loss of SOX9 led ependymal cells to adopt a neuroblast identity. These data identify a functional link between extrinsic and intrinsic mechanisms of NSCs specification and maintenance, and establish a central role for SOX9 in the process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Gage, F.H. Mammalian neural stem cells. Science 287, 1433–1438 (2000).
Temple, S. The development of neural stem cells. Nature 414, 112–117 (2001).
Spassky, N. et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 25, 10–18 (2005).
Doetsch, F. The glial identity of neural stem cells. Nat. Neurosci. 6, 1127–1134 (2003).
Reynolds, B.A. & Weiss, S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 175, 1–13 (1996).
Shi, Y., Sun, G., Zhao, C. & Stewart, R. Neural stem cell self-renewal. Crit. Rev. Oncol. Hematol. 65, 43–53 (2008).
Gulacsi, A.A. & Anderson, S.A. Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat. Neurosci. 11, 1383–1391 (2008).
Ahn, S. & Joyner, A.L. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437, 894–897 (2005).
Machold, R. et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 39, 937–950 (2003).
Lai, K., Kaspar, B.K., Gage, F.H. & Schaffer, D.V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 6, 21–27 (2003).
Rowitch, D.H. et al. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J. Neurosci. 19, 8954–8965 (1999).
Avilion, A.A. et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17, 126–140 (2003).
Conti, L. et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 3, e283 (2005).
Pevny, L. & Placzek, M. SOX genes and neural progenitor identity. Curr. Opin. Neurobiol. 15, 7–13 (2005).
Wood, H.B. & Episkopou, V. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech. Dev. 86, 197–201 (1999).
Favaro, R. et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 12, 1248–1256 (2009).
Takanaga, H. et al. Gli2 Is A novel regulator of Sox2 expression in telencephalic neuroepithelial cells. Stem Cells 27, 165–174 (2009).
Stolt, C.C. & Wegner, M. SoxE function in vertebrate nervous system development. Int. J. Biochem. Cell Biol. 42, 437–440 (2010).
Cheung, M. & Briscoe, J. Neural crest development is regulated by the transcription factor Sox9. Development 130, 5681–5693 (2003).
Vidal, V.P. et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 15, 1340–1351 (2005).
Kordes, U., Cheng, Y.C. & Scotting, P.J. Sox group E gene expression distinguishes different types and maturational stages of glial cells in developing chick and mouse. Brain Res. Dev. Brain Res. 157, 209–213 (2005).
Sottile, V., Li, M. & Scotting, P.J. Stem cell marker expression in the Bergmann glia population of the adult mouse brain. Brain Res. 1099, 8–17 (2006).
Cheng, L.C., Pastrana, E., Tavazoie, M. & Doetsch, F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 12, 399–408 (2009).
Stolt, C.C. et al. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 17, 1677–1689 (2003).
Stamataki, D., Ulloa, F., Tsoni, S.V., Mynett, A. & Briscoe, J. A gradient of Gli activity mediates graded Sonic Hedgehog signaling in the neural tube. Genes Dev. 19, 626–641 (2005).
Kempermann, G., Gast, D., Kronenberg, G., Yamaguchi, M. & Gage, F.H. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 130, 391–399 (2003).
Tronche, F. et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23, 99–103 (1999).
Akiyama, H. et al. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc. Natl. Acad. Sci. USA 101, 6502–6507 (2004).
Houston, C.S. et al. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al in 1971. Am. J. Med. Genet. 15, 3–28 (1983).
Palma, V. & Ruiz i Altaba, A. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development 131, 337–345 (2004).
Anton, M. & Graham, F.L. Site-specific recombination mediated by an adenovirus vector expressing the Cre recombinase protein: a molecular switch for control of gene expression. J. Virol. 69, 4600–4606 (1995).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Akiyama, H., Chaboissier, M.C., Martin, J.F., Schedl, A. & de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813–2828 (2002).
Carlén, M. et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 12, 259–267 (2009).
Bien-Willner, G.A., Stankiewicz, P. & Lupski, J.R. SOX9cre1, a cis-acting regulatory element located 1.1 Mb upstream of SOX9, mediates its enhancement through the SHH pathway. Hum. Mol. Genet. 16, 1143–1156 (2007).
Cheung, M. et al. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell 8, 179–192 (2005).
Mizutani, K., Yoon, K., Dang, L., Tokunaga, A. & Gaiano, N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355 (2007).
Kalani, M.Y. et al. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc. Natl. Acad. Sci. USA 105, 16970–16975 (2008).
Bonaguidi, M.A. et al. Noggin expands neural stem cells in the adult hippocampus. J. Neurosci. 28, 9194–9204 (2008).
Nowak, J.A., Polak, L., Pasolli, H.A. & Fuchs, E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3, 33–43 (2008).
Poché, R.A., Furuta, Y., Chaboissier, M.C., Schedl, A. & Behringer, R.R. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Muller glial cell development. J. Comp. Neurol. 510, 237–250 (2008).
Okubo, T., Knoepfler, P.S., Eisenman, R.N. & Hogan, B.L. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development 132, 1363–1374 (2005).
Takaki, M., Nakayama, S., Misawa, H., Nakagawa, T. & Kuniyasu, H. In vitro formation of enteric neural network structure in a gut-like organ differentiated from mouse embryonic stem cells. Stem Cells 24, 1414–1422 (2006).
Miller, S.J. et al. Large-scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res. 66, 2584–2591 (2006).
Kordes, U. & Hagel, C. Expression of SOX9 and SOX10 in central neuroepithelial tumor. J. Neurooncol. 80, 151–155 (2006).
Novak, A., Guo, C., Yang, W., Nagy, A. & Lobe, C.G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147–155 (2000).
Nagy, A., Rossant, J., Nagy, R., Abramow-Newerly, W. & Roder, J.C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90, 8424–8428 (1993).
Briscoe, J., Chen, Y., Jessell, T.M. & Struhl, G. A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol. Cell 7, 1279–1291 (2001).
Doetsch, F., Caille, I., Lim, D.A. & Garcia-Verdugo, J.M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Gentleman, R.C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004).
Acknowledgements
We thank A. Schedl (INSERM U636, Nice) for the conditional Sox9 mutant mice, A. Nagy (Samuel Lunenfeld Research Institute) for the pCall2 vector, W.C.W. Chan for blastocyst injections and production of Z/Sox9 mice and T. Caspary (Emory University) for the Arl13b antibody. The CMV:cre construct was a gift from S. O'Gorman (Salk Institute). Thank you to S. Guioli, F. Guillemot and L. Reynard for critical reading of the manuscript, to C. Andoniadou for advice and training in the ways of neurosphere cultures, to W. Han Yau in the photographics department at NIMR for help with illustrations, to T. Matabanadzo and other biological services staff at NIMR for help with the mouse colonies and other members of our laboratories for discussion and encouragement. This work was supported by the UK Medical Research Council (U117512772), a US National Institutes of Health (National Institute of Biomedical Imaging and Bioengineering) Quantum Grant (R.L.-B. and C.E.S.), and grants from the Hong Kong Research Grants Council and the Hong Kong University Grants Council Area of Excellence Scheme (S.L.W. and K.S.E.C.).
Author information
Authors and Affiliations
Contributions
C.E.S., J.B. and R.L.-B. initiated the project. C.E.S. performed the in ovo electroporations, neurosphere culturing, immunohistochemistry, RT-PCR and quantification and data analysis of all the in vivo and in vitro experiments, except for the acquisition and analysis of the microarray data (C.C.). S.L.W., B.G. and K.S.E.C. generated the Z/Sox9 mice. M.C. supplied many of the constructs used in this study. A.S. performed the adenovirus injections and S.B. perfused the adult mice. M.-V.G.G. carried out the BrdU injections. C.E.S., R.L.-B. and J.B. were involved in the study design and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 (PDF 1910 kb)
Rights and permissions
About this article
Cite this article
Scott, C., Wynn, S., Sesay, A. et al. SOX9 induces and maintains neural stem cells. Nat Neurosci 13, 1181–1189 (2010). https://doi.org/10.1038/nn.2646
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2646
This article is cited by
-
Antiproliferative and apoptotic effects of telmisartan in human glioma cells
Cancer Cell International (2023)
-
Post-transcriptional control of a stemness signature by RNA-binding protein MEX3A regulates murine adult neurogenesis
Nature Communications (2023)
-
miR-124-3p regulates the proliferation and differentiation of retinal progenitor cells through SEPT10
Cell and Tissue Research (2023)
-
Sex steroid hormone synthesis, metabolism, and the effects on the mammalian olfactory system
Cell and Tissue Research (2023)
-
Identifying Genes that Affect Differentiation of Human Neural Stem Cells and Myelination of Mature Oligodendrocytes
Cellular and Molecular Neurobiology (2023)