Abstract
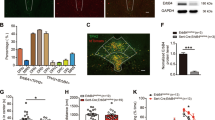
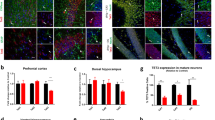
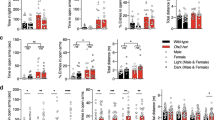
Transcriptional cascades are required for the specification of serotonin (5-HT) neurons and behaviors modulated by 5-HT. Several cascade factors are expressed throughout the lifespan, which suggests that their control of behavior might not be temporally restricted to programming normal numbers of 5-HT neurons. We used new mouse conditional targeting approaches to investigate the ongoing requirements for Pet-1 (also called Fev), a cascade factor that is required for the initiation of 5-HT synthesis, but whose expression persists into adulthood. We found that Pet-1 was required after the generation of 5-HT neurons for multiple steps in 5-HT neuron maturation, including axonal innervation of the somatosensory cortex, expression of appropriate firing properties, and the expression of the Htr1a and Htr1b autoreceptors. Pet-1 was still required in adult 5-HT neurons to preserve normal anxiety-related behaviors through direct autoregulated control of serotonergic gene expression. These findings indicate that Pet-1 is required across the lifespan of the mouse and that behavioral pathogenesis can result from both developmental and adult-onset alterations in serotonergic transcription.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Holmes, A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci. Biobehav. Rev. 32, 1293–1314 (2008).
Ansorge, M.S., Hen, R. & Gingrich, J.A. Neurodevelopmental origins of depressive disorders. Curr. Opin. Pharmacol. 7, 8–17 (2007).
Vitalis, T., Cases, O., Passemard, S., Callebert, J. & Parnavelas, J.G. Embryonic depletion of serotonin affects cortical development. Eur. J. Neurosci. 26, 331–344 (2007).
Salichon, N. et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J. Neurosci. 21, 884–896 (2001).
Bonnin, A., Torii, M., Wang, L., Rakic, P. & Levitt, P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat. Neurosci. 10, 588–597 (2007).
Ansorge, M.S., Zhou, M., Lira, A., Hen, R. & Gingrich, J.A. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306, 879–881 (2004).
Maciag, D. et al. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology 31, 47–57 (2006).
Gross, C. et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416, 396–400 (2002).
Jans, L.A., Riedel, W.J., Markus, C.R. & Blokland, A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol. Psychiatry 12, 522–543 (2007).
Scott, M.M. & Deneris, E.S. Making and breaking serotonin neurons and autism. Int. J. Dev. Neurosci. 23, 277–285 (2005).
Cordes, S.P. Molecular genetics of the early development of hindbrain serotonergic neurons. Clin. Genet. 68, 487–494 (2005).
Hendricks, T.J. et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37, 233–247 (2003).
Dai, J.X. et al. Enhanced contextual fear memory in central serotonin-deficient mice. Proc. Natl. Acad. Sci. USA 105, 11981–11986 (2008).
Hendricks, T., Francis, N., Fyodorov, D. & Deneris, E. The ETS domain factor Pet-1 is an early and precise marker of central 5-HT neurons and interacts with a conserved element in serotonergic genes. J. Neurosci. 19, 10348–10356 (1999).
Scott, M.M. et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc. Natl. Acad. Sci. USA 102, 16472–16477 (2005).
Jacob, J. et al. Transcriptional repression coordinates the temporal switch from motor to serotonergic neurogenesis. Nat. Neurosci. 10, 1433–1439 (2007).
Pattyn, A. et al. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 17, 729–737 (2003).
Zhao, Z.Q. et al. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J. Neurosci. 26, 12781–12788 (2006).
Krueger, K.C. & Deneris, E.S. Serotonergic transcription of human FEV reveals direct GATA factor interactions and fate of Pet-1-deficient serotonin neuron precursors. J. Neurosci. 28, 12748–12758 (2008).
Lidov, H.G. & Molliver, M.E. An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Res. Bull. 8, 389–430 (1982).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Vertes, R.P., Fortin, W.J. & Crane, A.M. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 407, 555–582 (1999).
Le Francois, B., Czesak, M., Steubl, D. & Albert, P.R. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology 55, 977–985 (2008).
Fornal, C.A. et al. Single-unit responses of serotonergic dorsal raphe neurons to 5–HT1A agonist and antagonist drug administration in behaving cats. J. Pharmacol. Exp. Ther. 270, 1345–1358 (1994).
Bayliss, D.A., Li, Y.W. & Talley, E.M. Effects of serotonin on caudal raphe neurons: activation of an inwardly rectifying potassium conductance. J. Neurophysiol. 77, 1349–1361 (1997).
Sari, Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci. Biobehav. Rev. 28, 565–582 (2004).
Bonnin, A., Peng, W., Hewlett, W. & Levitt, P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience 141, 781–794 (2006).
Craven, S.E. et al. Gata2 specifies serotonergic neurons downstream of sonic hedgehog. Development 131, 1165–1173 (2004).
Zhu, J. et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat. Immunol. 5, 1157–1165 (2004).
Feil, R., Wagner, J., Metzger, D. & Chambon, P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 237, 752–757 (1997).
Vogt, M.A. et al. Suitability of tamoxifen-induced mutagenesis for behavioral phenotyping. Exp. Neurol. 211, 25–33 (2008).
Scott, M.M., Krueger, K.C. & Deneris, E.S. A differentially autoregulated Pet-1 enhancer region is a critical target of the transcriptional cascade that governs serotonin neuron development. J. Neurosci. 25, 2628–2636 (2005).
Janusonis, S., Gluncic, V. & Rakic, P. Early serotonergic projections to Cajal-Retzius cells: relevance for cortical development. J. Neurosci. 24, 1652–1659 (2004).
Gutknecht, L. et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J. Neural Transm. 115, 1127–1132 (2008).
Ludwig, V. & Schwarting, R.K. Neurochemical and behavioral consequences of striatal injection of 5,7-dihydroxytryptamine. J. Neurosci. Methods 162, 108–118 (2007).
Pum, M.E., Huston, J.P. & Muller, C.P. The role of cortical serotonin in anxiety and locomotor activity in Wistar rats. Behav. Neurosci. 123, 449–454 (2009).
Sommer, W. et al. Local 5,7-dihydroxytryptamine lesions of rat amygdala: release of punished drinking, unaffected plus-maze behavior and ethanol consumption. Neuropsychopharmacology 24, 430–440 (2001).
Varga, V. et al. Fast synaptic subcortical control of hippocampal circuits. Science 326, 449–453 (2009).
Heisler, L.K. et al. Elevated anxiety and antidepressant-like responses in serotonin 5–HT1A receptor mutant mice. Proc. Natl. Acad. Sci. USA 95, 15049–15054 (1998).
Ramboz, S. et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc. Natl. Acad. Sci. USA 95, 14476–14481 (1998).
Richardson-Jones, J.W. et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65, 40–52 (2010).
Flames, N. & Hobert, O. Gene regulatory logic of dopamine neuron differentiation. Nature 458, 885–889 (2009).
Hobert, O. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc. Natl. Acad. Sci. USA 105, 20067–20071 (2008).
Gardner, K.L., Hale, M.W., Lightman, S.L., Plotsky, P.M. & Lowry, C.A. Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Res. 1305, 47–63 (2009).
Shishkina, G.T., Kalinina, T.S. & Dygalo, N.N. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience 150, 404–412 (2007).
Lerch-Haner, J.K., Frierson, D., Crawford, L.K., Beck, S.G. & Deneris, E.S. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat. Neurosci. 11, 1001–1003 (2008).
Rivera, H.M., Oberbeck, D.R., Kwon, B., Houpt, T.A. & Eckel, L.A. Estradiol increases Pet-1 and serotonin transporter mRNA in the midbrain raphe nuclei of ovariectomized rats. Brain Res. 1259, 51–58 (2009).
O'Gorman, S., Dagenais, N.A., Qian, M. & Marchuk, Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl. Acad. Sci. USA 94, 14602–14607 (1997).
Acknowledgements
We thank L. Landmesser for suggestions on retrograde tracing; S. Dymecki and P. Chambon for CreERT2 plasmids; Q. Ma for the mycPet-1 vector; J. Zhu for the Gata3loxP/loxP mice; K. Lobur for assistance with genotyping of mice; J. Reeves for behavioral testing in the Case Rodent Behavior Core; and L. Landmesser, S. Maricich and J. Silver for comments on the manuscript. This work was supported by grants MH062723 and MH078028 to E.S.D. (US National Institutes of Health).
Author information
Authors and Affiliations
Contributions
E.S.D. conceived the project. C.L. made the transgenic and targeting constructs, characterized all new mouse lines and generated all histological, RT-PCR and retrograde tracing data and images. C.L. and S.C.W. performed western blot analyses. C.L. and G.C. performed behavioral analyses. T.M. and S.H. generated the electrophysiology data. C.L., S.H., T.M., G.C., S.C.W. and E.S.D. analyzed the data. E.S.D. and C.L. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Tables 1 and 2 (PDF 1026 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Maejima, T., Wyler, S. et al. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci 13, 1190–1198 (2010). https://doi.org/10.1038/nn.2623
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2623
This article is cited by
-
Transcriptomic and epigenomic analyses explore the potential role of H3K4me3 in neomycin-induced cochlear Lgr5+ progenitor cell regeneration of hair cells
Human Cell (2022)
-
Serotonin deficiency induced after brain maturation rescues consequences of early life adversity
Scientific Reports (2021)
-
Pten is a key intrinsic factor regulating raphe 5-HT neuronal plasticity and depressive behaviors in mice
Translational Psychiatry (2021)
-
Embracing diversity in the 5-HT neuronal system
Nature Reviews Neuroscience (2019)
-
Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice
Communications Biology (2019)