Abstract
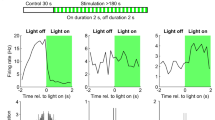
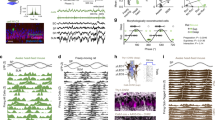
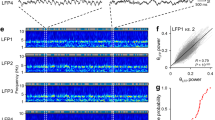
Although hippocampal theta oscillations represent a prime example of temporal coding in the mammalian brain, little is known about the specific biophysical mechanisms. Intracellular recordings support a particular abstract oscillatory interference model of hippocampal theta activity, the soma-dendrite interference model. To gain insight into the cellular and circuit level mechanisms of theta activity, we implemented a similar form of interference using the actual hippocampal network in mice in vitro. We found that pairing increasing levels of phasic dendritic excitation with phasic stimulation of perisomatic projecting inhibitory interneurons induced a somatic polarization and action potential timing profile that reproduced most common features. Alterations in the temporal profile of inhibition were required to fully capture all features. These data suggest that theta-related place cell activity is generated through an interaction between a phasic dendritic excitation and a phasic perisomatic shunting inhibition delivered by interneurons, a subset of which undergo activity-dependent presynaptic modulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Buzsaki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002).
Salinas, E. & Sejnowski, T. Correlated neuronal activity and the flow of neuronal information. Nat. Rev. Neurosci. 2, 539–550 (2001).
Laurent, G. & Davidowitz, H. Encoding of olfactory information with oscillating assemblies. Science 265, 1872–1875 (1994).
Bland, B.H. Physiology and Pharmacology of hippocampal formation theta rhythms. Prog. Neurobiol. 26, 1–54 (1986).
Margrie, T.W. & Schaefer, A.T. Theta oscillation coupled spike latencies yield computational vigor in a mammalian sensory system. J. Physiol. (Lond.) 546, 363–374 (2003).
O'Keefe, J. & Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 (1993).
Skaggs, W.E., McNaughton, B.L., Wilson, M.A. & Barnes, C.A. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172 (1996).
Hopfield, J.J. Pattern recognition computation using action potential timing for stimulus representation. Nature 376, 33–36 (1995).
Harris, K.D. et al. Spike train dynamics predicts theta-related phase precession in hippocampal pyramidal cells. Nature 417, 738–741 (2002).
Mehta, M.R., Lee, A.K. & Wilson, M.A. Role of experience and oscillations in transforming a rate code into a temporal code. Nature 417, 741–746 (2002).
O'Keefe, J. & Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971).
Harvey, C.D., Collman, F., Dombeck, D.A. & Tank, D.W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946 (2009).
Maurer, A.P. & McNaughton, B.L. Network and intrinsic cellular mechanisms underlying theta phase precession of hippocampal neurons. Trends Neurosci. 30, 325–333 (2007).
Lengyel, M., Szatmary, Z. & Erdi, P. Dynamically detuned oscillations account for the coupled rate and temporal code of place cell firing. Hippocampus 13, 700–714 (2003).
Kamondi, A., Acsady, L., Wang, X.J. & Buzsaki, G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus 8, 244–261 (1998).
Magee, J.C. Dendritic mechanisms of phase precession in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 86, 528–532 (2001).
Wallenstein, G.V. & Hasselmo, M.E. GABAergic modulation of hippocampal population activity: sequence learning, place field development and the phase precession effect. J. Neurophysiol. 78, 393–408 (1997).
Bose, A., Booth, V. & Recce, M. A temporal mechanism for generating the phase precession of hippocampal place cells. J. Comput. Neurosci. 9, 5–30 (2000).
Tsodyks, M.V., Skaggs, W.E., Sejnowski, T.J. & McNaughton, B.L. Population dynamics and theta rhythm phase precession of hippocampal place cell firing: a spiking neuron model. Hippocampus 6, 271–280 (1996).
Jensen, O. & Lisman, J.E. Hippocampal CA3 region predicts memory sequences: accounting for the phase precession of place cells. Learn. Mem. 3, 279–287 (1996).
Atasoy, D., Aponte, Y., Su, H.H. & Sternson, S.M.A. FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008).
Hippenmeyer, S. et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 3, e159 (2005).
Andrasfalvy, B., Zemelman, B., Tang, J. & Vaziri, A. Two-photon optogenetic control of neural activity with single synapse precession by sculpted light. Proc. Natl. Acad. Sci. USA published online, doi:10.1073/pnas.1006620107 (11 June 2010).
Chance, F.S., Abbott, L. & Reyes, A. Gain modulation from background synaptic input. Neuron 35, 773–782 (2002).
Mitchell, S.J. & Silver, R.A. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38, 433–445 (2003).
Takahashi, H. & Magee, J.C. Pathway interactions and synaptic plasticity in the distal tuft regions of CA1 pyramidal neurons. Neuron 62, 102–111 (2009).
McNaughton, B.L., Barnes, C.A. & O'Keefe, J. The contributions of position, direction and velocity to single-unit activity in the hippocampus of freely moving rats. Exp. Brain Res. 52, 41–49 (1983).
O'Keefe, J. & Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 (1993).
Redish, D.A. et al. Independence of firing correlates of anatomically proximate hippocampal pyramidal cells. J. Neurosci. 21, 1–6 (2001).
Katona, I. et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 19, 4544–4558 (1999).
Wilson, R.I. & Nicoll, R.A. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature 410, 588–592 (2001).
Losonczy, A., Biro, A.A. & Nusser, Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc. Natl. Acad. Sci. USA 101, 1362–1367 (2004).
Klausberger, T. et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J. Neurosci. 25, 9782–9793 (2005).
Freund, T.F. & Katona, I. Perisomatic inhibition. Neuron 56, 33–42 (2007).
Hefft, S. & Jonas, P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat. Neurosci. 8, 1319–1328 (2005).
Ali, A.B. & Todorova, M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur. J Neurosci. 31, 1196–1207 (2010).
Robbe, D. & Buzsáki, G. Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. J. Neurosci. 29, 12597–12605 (2009).
Robbe, D. et al. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat. Neurosci. 9, 1526–1533 (2006).
Schmidt, R. et al. Single-trial phase precession in the hippocampus. J. Neurosci. 29, 13232–13241 (2009).
Dave, A.S. & Margoliash, D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science 290, 812–816 (2000).
Kleinfeld, D., Berg, R.W. & O'Connor, S.M. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens. Mot. Res. 16, 69–88 (1999).
Mizuseki, K., Sirota, A., Pastalkova, E. & Buzsaki, G. Theta oscillations provide temporal windows for the local circuit computation in the entorhinal-hippocampal loop. Neuron 64, 267–280 (2009).
Lisman, J.E. & Grace, A.A. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46, 703–713 (2005).
Hasselmo, M.E. & Eichenbaum, H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 18, 1172–1190 (2005).
Somogyi, P., Tamás, G., Lujan, R. & Buhl, E.H. Salient features of synaptic organization in the cerebral cortex. Brain Res. Brain Res. Rev. 26, 113–135 (1998).
Royer, S. et al. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur. J. Neurosci. 31, 2279–2291 (2010).
Grieger, J.C., Choi, V.W. & Samulski, R.J. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 1, 1412–1428 (2007).
Losonczy, A. & Magee, J.C. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron 50, 291–307 (2006).
Vaziri, A., Tang, J., Shroff, H. & Shank, C. Multilayer three-dimensional super-resolution imaging of thick biological samples. Proc. Natl. Acad. Sci. USA 105, 20221–20226 (2008).
Acknowledgements
We thank B. Shield, D. O'Connor and A. Villacis for help with histology and stereotaxic viral injections, and B.K. Andrasfalvy for help with the temporal focusing experiments. We thank for P. Somogyi and S. Siegelbaum for their comments on a previous version of the manuscript. Precursors of the GAD65-Cre knock-in mice were originally developed by B.V.Z. in the laboratory of G. Miesenboeck at the Memorial Sloan-Kettering Cancer Center in New York.
Author information
Authors and Affiliations
Contributions
A.L. and J.C.M. performed electrophysiological experiments, analyzed the data and wrote the paper. B.V.Z. prepared plasmids, designed Cre recombinase–dependent rAAV-FLEX-rev-ChR2-GFP viruses, generated the GAD65-Cre knock-in mouse line and helped with the manuscript. A.V. designed and built the experimental setup for temporal focusing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 4166 kb)
Rights and permissions
About this article
Cite this article
Losonczy, A., Zemelman, B., Vaziri, A. et al. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. Nat Neurosci 13, 967–972 (2010). https://doi.org/10.1038/nn.2597
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2597
This article is cited by
-
Homogeneous inhibition is optimal for the phase precession of place cells in the CA1 field
Journal of Computational Neuroscience (2023)
-
The role of inhibitory circuits in hippocampal memory processing
Nature Reviews Neuroscience (2022)
-
Multimodal determinants of phase-locked dynamics across deep-superficial hippocampal sublayers during theta oscillations
Nature Communications (2020)
-
Active dendritic integration as a mechanism for robust and precise grid cell firing
Nature Neuroscience (2017)
-
Three-dimensional spatiotemporal focusing of holographic patterns
Nature Communications (2016)