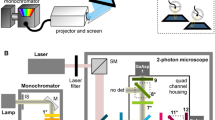
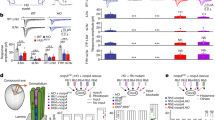
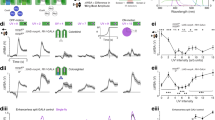
Abstract
In the visual system of Drosophila, photoreceptors R1–R6 relay achromatic brightness information to five parallel pathways. Two of them, the lamina monopolar cells L1 and L2, represent the major input lines to the motion detection circuitry. We devised a new method for optical recording of visually evoked changes in intracellular Ca2+ in neurons using targeted expression of a genetically encoded Ca2+ indicator. Ca2+ in single terminals of L2 neurons in the medulla carried no information about the direction of motion. However, we found that brightness decrements (light-OFF) induced a strong increase in intracellular Ca2+ but brightness increments (light-ON) induced only small changes, suggesting that half-wave rectification of the input signal occurs. Thus, L2 predominantly transmits brightness decrements to downstream circuits that then compute the direction of image motion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tammero, L.F. & Dickinson, M.H. The influence of visual landscape on the free flight behavior of the fruit fly Drosophila melanogaster. J. Exp. Biol. 205, 327–343 (2002).
Heisenberg, M. & Wolf, R. On the structure of yaw torque in visual flight orientation of Drosophila melanogaster. J. Comp. Physiol. [A] 130, 113–130 (1979).
Tammero, L.F. & Dickinson, M.H. Collision-avoidance and landing responses are mediated by separate pathways in the fruit fly, Drosophila melanogaster. J. Exp. Biol. 205, 2785–2798 (2002).
Mronz, M. & Lehmann, F.O. The free-flight response of Drosophila to motion of the visual environment. J. Exp. Biol. 211, 2026–2045 (2008).
Borst, A. & Haag, J. Neural networks in the cockpit of the fly. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 188, 419–437 (2002).
Clifford, C.W. & Ibbotson, M.R. Fundamental mechanisms of visual motion detection: models, cells and functions. Prog. Neurobiol. 68, 409–437 (2002).
Rister, J. et al. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron 56, 155–170 (2007).
Coombe, P.E. & Heisenberg, M. The structural brain mutant vacuolar medulla of Drosophila melanogaster with specific behavioral defects and cell degeneration in the adult. J. Neurogenet. 3, 135–158 (1986).
Zhu, Y., Nern, A., Zipursky, S.L. & Frye, M.A. Peripheral visual circuits functionally segregate motion and phototaxis behaviors in the fly. Curr. Biol. 19, 613–619 (2009).
Katsov, A.Y. & Clandinin, T.R. Motion processing streams in Drosophila are behaviorally specialized. Neuron 59, 322–335 (2008).
Hassenstein, B. & Reichardt, W. Systemtheoretische Analyse einer Verhaltensweise (der Wechsel-Folgen-Reaktion des Rüsselkäfers Chlorophanus viridis). Verh. Dtsch. Zool. Ges. 7, 95–102 (1952).
Hassenstein, B. & Reichardt, W. Systemtheoretische Analyse der Zeit-, Reihenfolgen- und Vorzeichenauswertung bei der Bewegungsperzeption des Rüsselkäfers Chlorophanus. Z. Naturforsch. [B] 11b, 513–524 (1956).
Götz, K.G. Optomotorische Untersuchungen des visuellen Systems einiger Augenmutanten der Fruchtfliege Drosophila. Kybernetik 2, 77–92 (1964).
van Doorn, A.J. & Koenderink, J.J. Temporal properties of the visual detectability of moving spatial white noise. Exp. Brain Res. 45, 179–188 (1982).
Barlow, H.B. & Levick, W.R. The mechanism of directionally selective units in rabbit's retina. J. Physiol. (Lond.) 178, 477–504 (1965).
van Santen, J.P.H. & Sperling, G. Temporal covariance model of human motion perception. J. Opt. Soc. Am. A 1, 451–473 (1984).
Adelson, E.H. & Bergen, J.R. Spatiotemporal energy models for the perception of motion. J. Opt. Soc. Am. A 2, 284–299 (1985).
Borst, A., Haag, J. & Reiff, D.F. Fly motion vision. Annu. Rev. Neurosci. 33, 49–70 (2010).
Joesch, M., Plett, J., Borst, A. & Reiff, D.F. Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr. Biol. 18, 368–374 (2008).
Schnell, B. et al. Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J. Neurophysiol. 103, 1646–1657 (2010).
Egelhaaf, M. & Borst, A. Are there separate ON and OFF channels in fly motion vision? Vis. Neurosci. 8, 151–164 (1992).
Franceschini, N., Riehle, A. & Le Nestour, A. Directionally selective motion detection by insect neurons. in Facets of Vision (eds. Stavenga, D.G. & Hardie, R.C.) Ch. 17, 360–390 (Springer-Verlag, Berlin, 1989).
Riehle, A. & Franceschini, N. Motion detection in flies: parametric control over ON-OFF pathways. Exp. Brain Res. 54, 390–394 (1984).
Ullman, S. The Interpretation of Visual Motion (MIT Press, Cambridge, Massachusetts, 1979).
Anstis, S.M. & Rogers, B.J. Illusory reversals of visual depth and movement during changes in contrast. Vision Res. 15, 957–961 (1975).
Takemura, S.Y., Lu, Z. & Meinertzhagen, I.A. Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. J. Comp. Neurol. 509, 493–513 (2008).
Strausfeld, N.J. Atlas of an Insect Brain (Springer, Berlin, 1976).
Fischbach, K.F. & Dittrich, A.P.M. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res. 258, 441–475 (1989).
Meinertzhagen, I.A. & O'Neil, S.D. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J. Comp. Neurol. 305, 232–263 (1991).
Gengs, C. et al. The target of Drosophila photoreceptor synaptic transmission is a histamine-gated chloride channel encoded by ort (hclA). J. Biol. Chem. 277, 42113–42120 (2002).
Hardie, R.C. A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature 339, 704–706 (1989).
Skingsley, D.R., Laughlin, S.B. & Hardie, R.C. Properties of histamine-activated chloride channels in the large monopolar cells of the dipteran compound eye: a comparative study. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 176, 611–623 (1995).
Laughlin, S.B. & Osorio, D. Mechanism for neural signal enhancement in the blowfly compound eye. J. Exp. Biol. 144, 113–146 (1989).
Järvilehto, M. & Zettler, F. Electrophysiological-histological studies on some functional properties of visual cells and second order neurons of an insect retina. Z. Zellforsch. Mikrosk. Anat. 136, 291–306 (1973).
Zettler, F. & Järvilehto, M. Lateral inhibition in an insect eye. Z. Vgl. Physiol. 76, 233–244 (1972).
Zettler, F. & Järvilehto, M. Active and passive axonal propagation of non-spike signals in the retina of Calliphora. J. Comp. Physiol. 85, 89–104 (1973).
Zettler, F. & Straka, H. Synaptic chloride channels generating hyperpolarizing on-responses in monopolar neurons of the blowfly visual system. J. Exp. Biol. 131, 435–438 (1987).
Zheng, L. et al. Feedback network controls photoreceptor output at the layer of first visual synapses in Drosophila. J. Gen. Physiol. 127, 495–510 (2006).
Douglass, J.K. & Strausfeld, N.J. Visual motion detection circuits in flies: peripheral motion computation by identified small-field retinotopic neurons. J. Neurosci. 15, 5596–5611 (1995).
Douglass, J.K. & Strausfeld, N.J. Visual motion-detection circuits in flies: parallel direction- and non-direction-sensitive pathways between the medulla and lobula plate. J. Neurosci. 16, 4551–4562 (1996).
Buschbeck, E.K. & Strausfeld, N.J. Visual motion-detection circuits in flies: small-field retinotopic elements responding to motion are evolutionarily conserved across taxa. J. Neurosci. 16, 4563–4578 (1996).
Mank, M. et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat. Methods 5, 805–811 (2008).
Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Euler, T., Detwiler, P.B. & Denk, W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418, 845–852 (2002).
Wu, C.F. & Wong, F. Frequency characteristics in the visual system of Drosophila: genetic dissection of electroretinogram components. J. Gen. Physiol. 69, 705–724 (1977).
Coombe, P.E. The large monopolar cells L1 and L2 are responsible for ERG transients in Drosophila. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 159, 655–665 (1986).
Wässle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 5, 747–757 (2004).
Molnar, A., Hsueh, H.A., Roska, B. & Werblin, F.S. Crossover inhibition in the retina: circuitry that compensates for nonlinear rectifying synaptic transmission. J. Comput. Neurosci. 27, 569–590 (2009).
Rentería, R.C. et al. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. J. Neurosci. 26, 11857–11869 (2006).
Chalasani, S.H. et al. Dissecting a circuit for olfactory behavior in Caenorhabditis elegans. Nature 450, 63–70 (2007).
Acknowledgements
We are very grateful to W. Denk, M. Mueller and J. Tritthardt for supporting and troubleshooting 2PLSM; M. Joesch for providing Matlab code and discussion; J. Haag and B. Schnell for discussion and comments on the manuscript; W. Essbauer and C. Theile for technical assistance; T. Gollisch for comments on the manuscript; G. Miesenböck and L. Sjulsion for their input on the use of LEDs; and the members of the mechanics and electronics workshop of the MPI Martinsried for excellent support.
Author information
Authors and Affiliations
Contributions
D.F.R. conceptualized the triggered stimulus presentation, J.P. designed and engineered the LED arena, M.M. and O.G. engineered TN-XXL, A.B. and D.F.R. designed experiments and wrote the manuscript, D.F.R. performed all of the fly work, imaging experiments and data analysis and prepared the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 154 kb)
Rights and permissions
About this article
Cite this article
Reiff, D., Plett, J., Mank, M. et al. Visualizing retinotopic half-wave rectified input to the motion detection circuitry of Drosophila. Nat Neurosci 13, 973–978 (2010). https://doi.org/10.1038/nn.2595
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2595
This article is cited by
-
Distinct expression of potassium channels regulates visual response properties of lamina neurons in Drosophila melanogaster
Journal of Comparative Physiology A (2020)
-
Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans
Nature Neuroscience (2019)
-
Evoking and tracking zebrafish eye movement in multiple larvae with ZebEyeTrack
Nature Protocols (2018)
-
Fly visual course control: behaviour, algorithms and circuits
Nature Reviews Neuroscience (2014)
-
A directional tuning map of Drosophila elementary motion detectors
Nature (2013)