Abstract
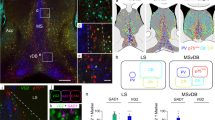
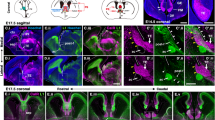
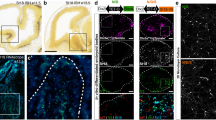
Neurons usually migrate and differentiate in one particular encephalic vesicle. We identified a murine population of diencephalic neurons that colonized the telencephalic amygdaloid complex, migrating along a tangential route that crosses a boundary between developing brain vesicles. The diencephalic transcription factor OTP was necessary for this migratory behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Swanson, L.W. & Petrovich, G.D. What is the amygdala? Trends Neurosci. 21, 323–331 (1998).
Alheid, G.F. & Heimer, L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid and corticopetal components of substantia innominata. Neuroscience 27, 1–39 (1988).
Cassell, M.D., Freedman, L.J. & Shi, C. The intrinsic organization of the central extended amygdala. Ann. NY Acad. Sci. 877, 217–241 (1999).
Shammah-Lagnado, S.J. et al. Supracapsular bed nucleus of the stria terminalis contains central and medial extended amygdala elements: evidence from anterograde and retrograde tracing experiments in the rat. J. Comp. Neurol. 422, 533–555 (2000).
Holmgren, N. Points of view concerning forebrain morphology in higher vertebrates. Acta Zool. 6, 413–447 (1925).
Swanson, L.W. Cerebral hemisphere regulation of motivated behavior. Brain Res. 886, 113–164 (2000).
Puelles, L. Brain segmentation and forebrain development in amniotes. Brain Res. Bull. 55, 695–710 (2001).
Martínez-García, F., Martínez-Marcos, A. & Lanuza, E. The pallial amygdala of amniote vertebrates: evolution of the concept, evolution of the structure. Brain Res. Bull. 57, 463–469 (2002).
Medina, L., Brox, A., Legaz, I., García-López, M. & Puelles, L. Expression patterns of developmental regulatory genes show comparable divisions in the telencephalon of Xenopus and mouse: insights into the evolution of the forebrain. Brain Res. Bull. 66, 297–302 (2005).
Tole, S., Remedios, R., Saha, B. & Stoykova, A. Selective requirement of Pax6, but not Emx2, in the specification and development of several nuclei of the amygdaloid complex. J. Neurosci. 25, 2753–2760 (2005).
Puelles, L. et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6 and Tbr-1. J. Comp. Neurol. 424, 409–438 (2000).
Medina, L. et al. Expression of Dbx1, Neurogenin 2, Semaphorin 5A, Cadherin 8 and Emx1 distinguish ventral and lateral pallial histogenetic divisions in the developing mouse claustroamygdaloid complex. J. Comp. Neurol. 474, 504–523 (2004).
Flames, N. et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 27, 9682–9695 (2007).
García-López, M. et al. Histogenetic compartments of the mouse centromedial and extended amygdala based on gene expression patterns during development. J. Comp. Neurol. 506, 46–74 (2008).
Canteras, N.S., Simerly, R.B. & Swanson, L.W. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J. Comp. Neurol. 360, 213–245 (1995).
Stenman, J., Toresson, H. & Campbell, K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J. Neurosci. 23, 167–174 (2003).
Wang, W. & Lufkin, T. The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev. Biol. 227, 432–449 (2000).
Bardet, S.M., Martínez-de-la-Torre, M., Northcutt, R.G., Rubenstein, J.L.R. & Puelles, L. Conserved pattern of OTP-positive cells in the paraventricular nucleus and other hypothalamic sites of tetrapods. Brain Res. Bull. 75, 231–235 (2008).
Michaud, J.L., Rosenquist, T., May, N.R. & Fan, C.M. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 12, 3264–3275 (1998).
Nakai, S. et al. The POU domain transcription factor Brn-2 is required for the determination of specific neuronal lineages in the hypothalamus of the mouse. Genes Dev. 9, 3109–3121 (1995).
Fan, C.M. et al. Expression patterns of two murine homologs of Drosophila single-minded suggest possible roles in embryonic patterning and in the pathogenesis of Down syndrome. Mol. Cell. Neurosci. 7, 1–16 (1996).
Sheng, H.Z. et al. Expression of murine Lhx5 suggests a role in specifying the forebrain. Dev. Dyn. 208, 266–277 (1997).
Puelles, L. & Rubenstein, J.L.R. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 26, 469–476 (2003).
Simeone, A. et al. Orthopedia, a novel homeobox-containing gene expressed in the developing CNS of both mouse and Drosophila. Neuron 13, 83–101 (1994).
Blechman, J. et al. Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia. Development 134, 4417–4426 (2007).
Acampora, D. et al. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 13, 2787–2800 (1999).
Acampora, D., Postiglione, M.P., Avantaggiato, V., Bonito, M.D. & Simeone, A. The role of Otx and Otp genes in brain development. Int. J. Dev. Biol. 44, 669–677 (2000).
Altman, J. & Bayer, S.A. Development of the diencephalon in the rat. I. Autoradiographic study of the time of origin and settling patterns of neurons of the hypothalamus. J. Comp. Neurol. 182, 945–971 (1978a).
Altman, J. & Bayer, S.A. Development of the diencephalon in the rat. II. Correlation of the embryonic development of the hypothalamus with the time of origin of its neurons. J. Comp. Neurol. 182, 973–993 (1978b).
Swanson, L.W. Development of the paraventricular nucleus of the hypothalamus. in From Development to Degeneration and Regeneration of the Nervous System (eds. Ribak, C.E., Arámburo-de la Hoz, C., Jones, E.G., Larriva-Sahd, J.A. & Swanson, L.W.) 69–84 (Oxford University Press, Oxford, 2009).
Ryu, S. et al. Orthopedia homeodomain protein is essential for diencephalic dopaminergic neuron development. Curr. Biol. 17, 873–880 (2007).
Choi, G.B. et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 46, 647–660 (2005).
Remedios, R., Subramanian, L. & Tole, S. LIM genes parcellate the embryonic amygdala and regulate its development. J. Neurosci. 24, 6986–6990 (2004).
Letinic, K. & Rakic, P. Telencephalic origin of human thalamic GABAergic neurons. Nat. Neurosci. 4, 931–936 (2001).
Rakic, P. Principles of neural cell migration. Experientia 46, 882–891 (1990).
De Carlos, J.A., López-Mascaraque, L. & Valverde, F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J. Neurosci. 16, 6146–6156 (1996).
Anderson, S., Mione, M., Yun, K. & Rubenstein, J.L. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb. Cortex 9, 646–654 (1999).
Lavdas, A.A., Grigoriou, M., Pachnis, V. & Parnavelas, J.G. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J. Neurosci. 19, 7881–7888 (1999).
Wichterle, H., Turnbull, D.H., Nery, S., Fishell, G. & Alvarez-Buylla, A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development 128, 3759–3771 (2001).
Zhao, T. et al. Genetic mapping of Foxb1 cell lineage shows migration from caudal diencephalon to telencephalon and lateral hypothalamus. Eur. J. Neurosci. 28, 1941–1955 (2008).
Soma, M. et al. Development of the mouse amygdala as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J. Comp. Neurol. 513, 113–128 (2009).
Hirata, T. et al. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat. Neurosci. 12, 141–149 (2009).
García-Moreno, F., López-Mascaraque, L. & De Carlos, J.A. Early telencephalic migration topographically converging in the olfactory cortex. Cereb. Cortex 18, 1239–1252 (2008).
Caqueret, A., Boucher, F. & Michaud, J.L. Laminar organization of the early developing anterior hypothalamus. Dev. Biol. 298, 95–106 (2006).
García-Moreno, F., López-Mascaraque, L. & De Carlos, J.A. Origins and migratory routes of murine Cajal-Retzius cells. J. Comp. Neurol. 500, 419–432 (2007).
Valverde, F. The Golgi method. A tool for comparative structural analyses. in Contemporary Research Methods in Neuroanatomy (eds Nauta, W.J.H. & Ebbensson, S.O.E.) 12–31 (Springer, New York, 1970).
Acknowledgements
We thank J. Lerma, A. Nieto, M.L. Ceci, A. Blanchart and J. García-Marqués for helpful comments; P. Bovolenta and C. Sánchez-Camacho for their kind gift of the pCAG plasmid; F. Vaccarino and G. Corte for the generous gift of antibodies to Otp and Foxg1; L. Menéndez de la Prida for providing the GAD-GFP transgenic mice; A. Kastanauskaite and L. María Suarez for their help with the intracellular injections of Lucifer Yellow and M. Sefton for editorial assistance. This work was supported by grants from the Ministerio de Ciencia e Innovación (BFU2007-60351/BFI), the OLFACTOSENSE Consortium of the Comunidad Autónoma de Madrid (P-SEM-0255-2006) and the Castilla-La Mancha Health Research Foundation (FISCAM PI2007-66) to J.A.DC., and the FP6 for the EUTRACC Integrate Project (LSHG-CT-2007-037445), the 'Stem Cell Project' of Fondazione Roma and the Italian Association for Cancer Research to A.S. Funding for M.P. was provided by Fundación Centro de Investigaciones de Enfermedades Neurológicas (PI006-08).
Author information
Authors and Affiliations
Contributions
J.A.D.C., L.L.-M. and F.G.-M. planned the experiments. F.G.-M. and M.P. carried out the experiments and analyzed the data. A.S., L.G.D.G. and M.D.S. performed and analyzed most of the experiments on Otp mutant mice. J.A.D.C. and F.G.-M. discussed the results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 10115 kb)
Supplementary Movie 1
Ecographic visualization of in utero injection. (MOV 1425 kb)
Rights and permissions
About this article
Cite this article
García-Moreno, F., Pedraza, M., Di Giovannantonio, L. et al. A neuronal migratory pathway crossing from diencephalon to telencephalon populates amygdala nuclei. Nat Neurosci 13, 680–689 (2010). https://doi.org/10.1038/nn.2556
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2556
This article is cited by
-
Quail-chick grafting experiments corroborate that Tbr1-positive eminential prethalamic neurons migrate along three streams into hypothalamus, subpallium and septocommissural areas
Brain Structure and Function (2021)
-
Development of the mouse anterior amygdalar radial unit marked by Lhx9-expression
Brain Structure and Function (2021)
-
Sim1-expressing cells illuminate the origin and course of migration of the nucleus of the lateral olfactory tract in the mouse amygdala
Brain Structure and Function (2021)
-
Neural pathways of olfactory kin imprinting and kin recognition in zebrafish
Cell and Tissue Research (2021)
-
Longitudinal developmental analysis of prethalamic eminence derivatives in the chick by mapping of Tbr1 in situ expression
Brain Structure and Function (2020)