Abstract
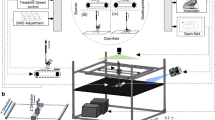
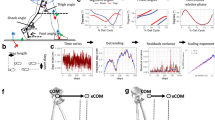
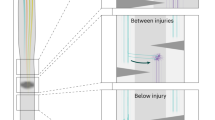
After complete spinal cord transections that removed all supraspinal inputs in adult rats, combinations of serotonergic agonists and epidural electrical stimulation were able to acutely transform spinal networks from nonfunctional to highly functional and adaptive states as early as 1 week after injury. Using kinematics, physiological and anatomical analyses, we found that these interventions could recruit specific populations of spinal circuits, refine their control via sensory input and functionally remodel these locomotor pathways when combined with training. The emergence of these new functional states enabled full weight-bearing treadmill locomotion in paralyzed rats that was almost indistinguishable from voluntary stepping. We propose that, in the absence of supraspinal input, spinal locomotion can emerge from a combination of central pattern-generating capability and the ability of these spinal circuits to use sensory afferent input to control stepping. These findings provide a strategy by which individuals with spinal cord injuries could regain substantial levels of motor control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kubasak, M.D. et al. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain 131, 264–276 (2008).
Ichiyama, R.M. et al. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J. Neurosci. 28, 7370–7375 (2008).
Courtine, G. et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 14, 69–74 (2008).
Grillner, S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52, 751–766 (2006).
Kiehn, O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 29, 279–306 (2006).
Gurfinkel, V.S., Levik, Y.S., Kazennikov, O.V. & Selionov, V.A. Locomotor-like movements evoked by leg muscle vibration in humans. Eur. J. Neurosci. 10, 1608–1612 (1998).
Dimitrijevic, M.R., Gerasimenko, Y. & Pinter, M. Evidence for a spinal central pattern generator in humans. Ann. NY Acad. Sci. 860, 360–376 (1998).
Chau, C., Barbeau, H. & Rossignol, S. Early locomotor training with clonidine in spinal cats. J. Neurophysiol. 79, 392–409 (1998).
Antri, M., Mouffle, C., Orsal, D. & Barthe, J.Y. 5–HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur. J. Neurosci. 18, 1963–1972 (2003).
Landry, E.S. et al. Contribution of spinal 5–HT1A and 5–HT7 receptors to locomotor-like movement induced by 8-OHDPAT in spinal cord–transected mice. Eur. J. Neurosci. 24, 535–546 (2006).
Ichiyama, R.M., Gerasimenko, Y.P., Zhong, H., Roy, R.R. & Edgerton, V.R. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 383, 339–344 (2005).
Gerasimenko, Y.P. et al. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J. Neurophysiol. 98, 2525–2536 (2007).
Guevremont, L. et al. Locomotor-related networks in the lumbosacral enlargement of the adult spinal cat: activation through intraspinal microstimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 14, 266–272 (2006).
Barthélemy, D., Leblond, H. & Rossignol, S. Characteristics and mechanisms of locomotion induced by intraspinal microstimulation and dorsal root stimulation in spinal cats. J. Neurophysiol. 97, 1986–2000 (2007).
De Leon, R.D., Hodgson, J.A., Roy, R.R. & Edgerton, V.R. Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J. Neurophysiol. 81, 85–94 (1999).
Tillakaratne, N.J. et al. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J. Neurosci. 22, 3130–3143 (2002).
Hochman, S., Garraway, S., Machacek, D. & Shay, B. 5-HT receptors and the neuromodulatory control of spinal cord function. in Motor Neurobiology of the Spinal Cord (ed. Cope, T.C.) 47–87 (CRC Press, Boca Raton, Florida, 2001).
Liu, J. & Jordan, L.M. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J. Neurophysiol. 94, 1392–1404 (2005).
Lavrov, I. et al. Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. J. Neurosci. 28, 6022–6029 (2008).
Petruska, J.C. et al. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J. Neurosci. 27, 4460–4471 (2007).
Côté, M.P. & Gossard, J.P. Step training–dependent plasticity in spinal cutaneous pathways. J. Neurosci. 24, 11317–11327 (2004).
Barthélemy, D., Leblond, H., Provencher, J. & Rossignol, S. Nonlocomotor and locomotor hindlimb responses evoked by electrical microstimulation of the lumbar cord in spinalized cats. J. Neurophysiol. 96, 3273–3292 (2006).
Lavrov, I. et al. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J. Neurophysiol. 96, 1699–1710 (2006).
Rossignol, S., Dubuc, R. & Gossard, J.P. Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 86, 89–154 (2006).
Pearson, K.G. Generating the walking gait: role of sensory feedback. Prog. Brain Res. 143, 123–129 (2004).
Barrière, G., Leblond, H., Provencher, J. & Rossignol, S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J. Neurosci. 28, 3976–3987 (2008).
Iwahara, T., Atsuta, Y., Garcia-Rill, E. & Skinner, R.D. Spinal cord stimulation-induced locomotion in the adult cat. Brain Res. Bull. 28, 99–105 (1992).
Gaunt, R.A., Prochazka, A., Mushahwar, V.K., Guevremont, L. & Ellaway, P.H. Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local alpha-motoneuron responses. J. Neurophysiol. 96, 2995–3005 (2006).
Rattay, F., Minassian, K. & Dimitrijevic, M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord. 2. Quantitative analysis by computer modeling. Spinal Cord 38, 473–489 (2000).
Rudomin, P. Central control of information transmission through the intraspinal arborizations of sensory fibers examined 100 years after Ramon y Cajal. Prog. Brain Res. 136, 409–421 (2002).
Giszter, S.F., Mussa-Ivaldi, F.A. & Bizzi, E. Convergent force fields organized in the frog's spinal cord. J. Neurosci. 13, 467–491 (1993).
Tresch, M.C. & Bizzi, E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low-threshold cutaneous stimulation. Exp. Brain Res. 129, 401–416 (1999).
Courtine, G. & Schieppati, M. Tuning of a basic coordination pattern constructs straight-ahead and curved walking in humans. J. Neurophysiol. 91, 1524–1535 (2004).
Ivanenko, Y.P., Cappellini, G., Dominici, N., Poppele, R.E. & Lacquaniti, F. Modular control of limb movements during human locomotion. J. Neurosci. 27, 11149–11161 (2007).
Lemay, M.A. & Grill, W.M. Modularity of motor output evoked by intraspinal microstimulation in cats. J. Neurophysiol. 91, 502–514 (2004).
Engesser-Cesar, C. et al. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur. J. Neurosci. 25, 1931–1939 (2007).
Harkema, S.J. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res. Rev. 57, 255–264 (2008).
Rioult-Pedotti, M.S., Friedman, D. & Donoghue, J.P. Learning-induced LTP in neocortex. Science 290, 533–536 (2000).
Dietz, V. & Muller, R. Degradation of neuronal function following a spinal cord injury: mechanisms and countermeasures. Brain 127, 2221–2231 (2004).
Harkema, S.J. et al. Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 77, 797–811 (1997).
Beres-Jones, J.A. & Harkema, S.J. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain 127, 2232–2246 (2004).
Lavrov, I. et al. Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input. J. Neurosci. 28, 7774–7780 (2008).
Bouyer, L.J. & Rossignol, S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J. Neurophysiol. 90, 3640–3653 (2003).
Grillner, S. & Wallen, P. Cellular bases of a vertebrate locomotor system-steering, intersegmental and segmental coordination and sensory control. Brain Res. Brain Res. Rev. 40, 92–106 (2002).
Fuentes, R., Petersson, P., Siesser, W.B., Caron, M.G. & Nicolelis, M.A. Spinal cord stimulation restores locomotion in animal models of Parkinson's disease. Science 323, 1578–1582 (2009).
Carhart, M.R., He, J., Herman, R., D'Luzansky, S. & Willis, W.T. Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 12, 32–42 (2004).
Courtine, G. et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 13, 561–566 (2007).
Maier, I.C. et al. Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain 132, 1426–1440 (2009).
Gerasimenko, Y.P. et al. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J. Neurosci. Methods 157, 253–263 (2006).
Courtine, G. et al. Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta). Brain 128, 2338–2358 (2005).
Acknowledgements
We would like to acknowledge the excellent technical help provided by S. Zdunowski, L. Friedli, J. Heutschi and O. Märzendorfer for data collection and analysis, as well as M. Herrera for his expert assistance and guidance involving the care and handling of the animals. This work was supported by the Craig H. Nielsen Foundation (#20062668), the International Paraplegic Foundation (P106), the National Center of Competence in Research 'Neural Plasticity and Repair' of the Swiss National Science Foundation, the Christopher and Dana Reeve Foundation (VEC-2007), the US National Institute of Neurological Disorders and Stroke (NS16333), the US Civilian Research and Development Foundation (RUB1-2872-07), the Roman Reed Spinal Cord Injury Research Fund of California and the Russian Foundation for Basic Research (07-04-00526 and 08-04-00688).
Author information
Authors and Affiliations
Contributions
G.C., Y.P.G., I.L., M.V.S. and V.R.E. designed the study. G.C., R.R.R., H.Z. and P.M. performed the surgeries. G.C., Y.P.G., R.v.d.B., P.M. and A.Y. carried out the experiments. G.C., A.Y., B.S., Y.A., R.I. and M.V.S. conducted the anatomical assessments. G.C. analyzed the data. G.C., R.R.R., M.V.S. and V.R.E. wrote the manuscript. G.C. supervised the study.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 1124 kb)
Supplementary Video 1
Accessing spinal locomotor circuits. (MOV 3260 kb)
Supplementary Video 2
Learning in spinal locomotor circuits. (MOV 8196 kb)
Supplementary Video 3
Control of spinal locomotor circuits. (MOV 3654 kb)
Rights and permissions
About this article
Cite this article
Courtine, G., Gerasimenko, Y., van den Brand, R. et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12, 1333–1342 (2009). https://doi.org/10.1038/nn.2401
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2401
This article is cited by
-
Epidural stimulation restores muscle synergies by modulating neural drives in participants with sensorimotor complete spinal cord injuries
Journal of NeuroEngineering and Rehabilitation (2023)
-
Longitudinal interrogation of sympathetic neural circuits and hemodynamics in preclinical models
Nature Protocols (2023)
-
Natural and targeted circuit reorganization after spinal cord injury
Nature Neuroscience (2022)
-
Minimal handgrip force is needed for transcutaneous electrical stimulation to improve hand functions of patients with severe spinal cord injury
Scientific Reports (2022)
-
A non-invasive system to monitor in vivo neural graft activity after spinal cord injury
Communications Biology (2022)