Abstract
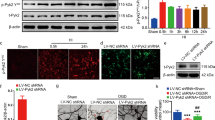
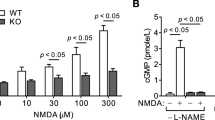
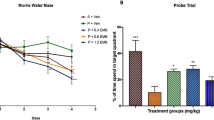
Cardiac arrest victims may experience transient brain hypoperfusion leading to delayed death of hippocampal CA1 neurons and cognitive impairment. We prevented this in adult rats by inhibiting the expression of transient receptor potential melastatin 7 (TRPM7), a transient receptor potential channel that is essential for embryonic development, is necessary for cell survival and trace ion homeostasis in vitro, and whose global deletion in mice is lethal. TRPM7 was suppressed in CA1 neurons by intrahippocampal injections of viral vectors bearing shRNA specific for TRPM7. This had no ill effect on animal survival, neuronal and dendritic morphology, neuronal excitability, or synaptic plasticity, as exemplified by robust long-term potentiation (LTP). However, TRPM7 suppression made neurons resistant to ischemic death after brain ischemia and preserved neuronal morphology and function. Also, it prevented ischemia-induced deficits in LTP and preserved performance in fear-associated and spatial-navigational memory tasks. Thus, regional suppression of TRPM7 is feasible, well tolerated and inhibits delayed neuronal death in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Hausenloy, D.J. & Scorrano, L. Targeting cell death. Clin. Pharmacol. Ther. 82, 370–373 (2007).
Volpe, B.T. & Petito, C.K. Dementia with bilateral medial temporal lobe ischemia. Neurology 35, 1793–1797 (1985).
Petito, C.K., Feldmann, E., Pulsinelli, W.A. & Plum, F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology 37, 1281–1286 (1987).
Bennett, M.V. et al. The GluR2 hypothesis: Ca2+-permeable AMPA receptors in delayed neurodegeneration. Cold Spring Harb. Symp. Quant. Biol. 61, 373–384 (1996).
Volpe, B.T., Pulsinelli, W.A., Tribuna, J. & Davis, H.P. Behavioral performance of rats following transient forebrain ischemia. Stroke 15, 558–562 (1984).
Block, F. Global ischemia and behavioral deficits. Prog. Neurobiol. 58, 279–295 (1999).
Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 79, 1431–1568 (1999).
Besancon, E., Guo, S., Lok, J., Tymianski, M. & Lo, E.H. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol. Sci. 29, 268–275 (2008).
Aarts, M. et al. A key role for TRPM7 channels in anoxic neuronal death. Cell 115, 863–877 (2003).
Montell, C., Birnbaumer, L. & Flockerzi, V. The TRP channels, a remarkably functional family. Cell 108, 595–598 (2002).
Monteilh-Zoller, M.K. et al. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 121, 49–60 (2003).
Nadler, M.J. et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595 (2001).
Schmitz, C. et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114, 191–200 (2003).
Jin, J. et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322, 756–760 (2008).
Siesjo, B.K., Katsura, K. & Tibor, K. Acidosis related brain damage. in Advances in Neurology: Cellular and Molecular Mechanisms of Ischemic Brain Damage (eds. Siesjo, B.K. & Wieloch, T.) (Raven Press, New York, 1994).
Silver, I.A. & Erecinska, M. Intracellular and exctracellular changes of [Ca2+] in hypoxia and ischemia in rat brain in vivo. J. Gen. Physiol. 95, 837–866 (1990).
Lin, M.C. et al. Microdialysis analyzer and flame atomic absorption spectrometry in the determination of blood glucose, lactate and magnesium in gerbils subjected to cerebral ischemia/reperfusion. J. Am. Coll. Nutr. 23, 556S–560S (2004).
Runnels, L.W., Yue, L. & Clapham, D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047 (2001).
Kozak, J.A., Kerschbaum, H.H. & Cahalan, M.D. Distinct properties of CRAC and MIC channels in RBL cells. J. Gen. Physiol. 120, 221–235 (2002).
Wei, W.L. et al. TRPM7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc. Natl. Acad. Sci. USA 104, 16323–16328 (2007).
Kaneko, S. et al. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J. Pharmacol. Sci. 101, 66–76 (2006).
Bridge, A.J., Pebernard, S., Ducraux, A., Nicoulaz, A.L. & Iggo, R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 34, 263–264 (2003).
Sledz, C.A., Holko, M., de Veer, M.J., Silverman, R.H. & Williams, B.R. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 5, 834–839 (2003).
Schmitz, C. et al. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J. Biol. Chem. 280, 37763–37771 (2005).
Xiong, Z.G., Chu, X.P. & MacDonald, J.F. Effect of lamotrigine on the Ca2+-sensing cation current in cultured hippocampal neurons. J. Neurophysiol. 86, 2520–2526 (2001).
Xiong, Z., Lu, W. & MacDonald, J.F. Extracellular calcium sensed by a novel cation channel in hippocampal neurons. Proc. Natl. Acad. Sci. USA 94, 7012–7017 (1997).
Pulsinelli, W.A. & Brierly, J.B. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 10, 267–272 (1979).
Pulsinelli, W.A., Brierly, J.B. & Plum, F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann. Neurol. 11, 491–498 (1982).
Saito, A. et al. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol. Neurobiol. 31, 105–116 (2005).
Corish, P. & Tyler-Smith, C. Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng. 12, 1035–1040 (1999).
Kumar, R. et al. Brain ischemia and reperfusion activates the eukaryotic initiation factor 2α kinase, PERK. J. Neurochem. 77, 1418–1421 (2001).
Stoll, G., Jander, S. & Schroeter, M. Inflammation and glial responses in ischemic brain lesions. Prog. Neurobiol. 56, 149–171 (1998).
Gavrieli, Y., Sherman, Y. & Ben-Sasson, S.A. Indentification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119, 493–501 (1992).
Ji, J. & Maren, S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus 17, 749–758 (2007).
Whitlock, J.R., Heynen, A.J., Shuler, M.G. & Bear, M.F. Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097 (2006).
Lisman, J., Schulman, H. & Cline, H. The molecular basis of CaMKII function in synaptic and behavioral memory. Nat. Rev. Neurosci. 3, 175–190 (2002).
Fanselow, M.S. Conditioned and unconditional components of post-shock freezing. Pavlov. J. Biol. Sci. 15, 177–182 (1980).
Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 (1984).
Morris, G.F. et al. Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The selfotel investigators. J. Neurosurg. 91, 737–743 (1999).
Davis, S.M., Albers, G.W., Diener, H.C., Lees, K.R. & Norris, J. Termination of acute stroke studies involving selfotel treatment. ASSIST steering committed. Lancet 349, 32 (1997).
Xiong, Z.G. et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118, 687–698 (2004).
Thompson, R.J., Zhou, N. & MacVicar, B.A. Ischemia opens neuronal gap junction hemichannels. Science 312, 924–927 (2006).
Lawlor, P.A. et al. Novel rat Alzheimer's disease models based on AAV-mediated gene transfer to selectively increase hippocampal Aβ levels. Mol. Neurodegener. 2, 11 (2007).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Academic Press, San Diego, 1998).
Mastakov, M.Y., Baer, K., Xu, R., Fitzsimons, H. & During, M.J. Combined injection of rAAV with mannitol enhances gene expression in the rat brain. Mol. Ther. 3, 225–232 (2001).
Sun, H.S., Feng, Z.P., Miki, T., Seino, S. & French, R.J. Enhanced neuronal damage after ischemic insults in mice lacking Kir6.2-containing ATP-sensitive K+ channels. J. Neurophysiol. 95, 2590–2601 (2006).
Mullen, R.J., Buck, C.R. & Smith, A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201–211 (1992).
Wang, L.Y. & MacDonald, J.F. Modulation by magnesium of the affinity of NMDA receptors for glycine in murine hippocampal neurones. J. Physiol. (Lond.) 486, 83–95 (1995).
Cheng, V.Y. et al. α5GABAA receptors mediate the amnestic, but not sedative-hypnotic, effects of the general anesthetic etomidate. J. Neurosci. 26, 3713–3720 (2006).
Acknowledgements
We thank M. Aarts and W. Czerwinski for technical information, J.C. Roder for advice on MWM testing, and A. Fleig, C. Montell, A. Scharenberg and E. Lo for a critical review of the manuscript. This work was supported by grants from the Canadian Institute of Health Research (MOP68939 and MOP89720 to M.T. and MOP15514 to J.F.M.), the US National Institutes of Health (NS048956 to M.T.), the Canadian Stroke Networks (M.T., J.F.M. and M.F.J.) and the Krembil Seed fund (M.T.). H.-S.S. is a recipient of Postdoctoral Fellowship Awards from the Heart and Stroke Foundation of Canada Focus on Stroke Training Initiative Program.
Author information
Authors and Affiliations
Contributions
H.-S.S. carried out the stereotactic rAAV infections, sectioning, immunochemistry, imaging, cell counts, laser dissection microcapture and PCR. K.J. and T.E.G. designed and manufactured the rAAV vectors, M.F.J. and J.F.M. performed the electrophysiology experiments, L.T. carried out the 4VO and histology procedures, Y.M. generated the antibodies to TRPM7, S.K. and H.C. performed the immunoblots, M.J. carried out the TUNEL cell counts, and J.P.F., M.J., H.C. and H.-S.S. performed the OGD experiments. L.J.M. and B.A.O. carried out the neurobehavioral evaluations. M.T. and H.-S.S. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11, Supplementary Table 1 and Supplementary Notes (PDF 6176 kb)
Rights and permissions
About this article
Cite this article
Sun, HS., Jackson, M., Martin, L. et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 12, 1300–1307 (2009). https://doi.org/10.1038/nn.2395
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2395
This article is cited by
-
Hypothermia increases adenosine monophosphate and xanthosine monophosphate levels in the mouse hippocampus, preventing their reduction by global cerebral ischemia
Scientific Reports (2024)
-
TRPM7 Mediates BSCB Disruption After Spinal Cord Injury by Regulating the mTOR/JMJD3 Axis in Rats
Molecular Neurobiology (2024)
-
Disseminated intravascular coagulation phenotype is regulated by the TRPM7 channel during sepsis
Biological Research (2023)
-
TRPM7 kinase mediates hypomagnesemia-induced seizure-related death
Scientific Reports (2023)
-
Hypoxia and interleukin-1-primed mesenchymal stem/stromal cells as novel therapy for stroke
Human Cell (2023)