Abstract
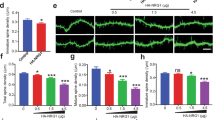
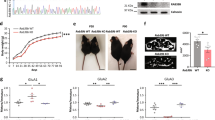
Dynamic remodeling of spiny synapses is crucial for cortical circuit development, refinement and plasticity, whereas abnormal morphogenesis is associated with neuropsychiatric disorders. We found that activation of Epac2, a PKA-independent cAMP target and Rap guanine-nucleotide exchange factor (GEF), in cultured rat cortical neurons induced spine shrinkage, increased spine motility, removed synaptic GluR2/3-containing AMPA receptors and depressed excitatory transmission, whereas its inhibition promoted spine enlargement and stabilization. Epac2 was required for dopamine D1-like receptor–dependent spine shrinkage and GluR2 removal from spines. Epac2 interaction with neuroligin promoted its membrane recruitment and enhanced its GEF activity. Rare missense mutations in the EPAC2 (also known as RAPGEF4) gene, previously found in individuals with autism, affected basal and neuroligin-stimulated GEF activity, dendritic Rap signaling, synaptic protein distribution and spine morphology. Thus, we identify a previously unknown mechanism that promotes dynamic remodeling and depression of spiny synapses, disruption of which may contribute to some aspects of disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others

References
Holtmaat, A.J. et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45, 279–291 (2005).
Kasai, H., Matsuzaki, M., Noguchi, J., Yasumatsu, N. & Nakahara, H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 26, 360–368 (2003).
Nägerl, U.V., Eberhorn, N., Cambridge, S.B. & Bonhoeffer, T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 44, 759–767 (2004).
Zhou, Q., Homma, K.J. & Poo, M.M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44, 749–757 (2004).
Oray, S., Majewska, A. & Sur, M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron 44, 1021–1030 (2004).
Pickett, J. & London, E. The neuropathology of autism: a review. J. Neuropathol. Exp. Neurol. 64, 925–935 (2005).
Tada, T. & Sheng, M. Molecular mechanisms of dendritic spine morphogenesis. Curr. Opin. Neurobiol. 16, 95–101 (2006).
Jordan, B.A. et al. Identification and verification of novel rodent postsynaptic density proteins. Mol. Cell. Proteomics 3, 857–871 (2004).
Peng, J. et al. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J. Biol. Chem. 279, 21003–21011 (2004).
Zhu, J.J., Qin, Y., Zhao, M., Van Aelst, L. & Malinow, R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110, 443–455 (2002).
Xie, Z., Huganir, R.L. & Penzes, P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron 48, 605–618 (2005).
Srivastava, D.P. et al. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc. Natl. Acad. Sci. USA 105, 14650–14655 (2008).
Zhu, Y. et al. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron 46, 905–916 (2005).
Morozov, A. et al. Rap1 couples cAMP signaling to a distinct pool of p42/44MAPK regulating excitability, synaptic plasticity, learning and memory. Neuron 39, 309–325 (2003).
Kawasaki, H. et al. A family of cAMP-binding proteins that directly activate Rap1. Science 282, 2275–2279 (1998).
Bos, J.L. Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 31, 680–686 (2006).
Bacchelli, E. et al. Screening of nine candidate genes for autism on chromosome 2q reveals rare non-synonymous variants in the cAMP-GEFII gene. Mol. Psychiatry 8, 916–924 (2003).
Abrahams, B.S. & Geschwind, D.H. Advances in autism genetics: on the threshold of a new neurobiology. Nat. Rev. Genet. 9, 341–355 (2008).
Chih, B., Afridi, S.K., Clark, L. & Scheiffele, P. Disorder-associated mutations lead to functional inactivation of neuroligins. Hum. Mol. Genet. 13, 1471–1477 (2004).
Chih, B., Engelman, H. & Scheiffele, P. Control of excitatory and inhibitory synapse formation by neuroligins. Science 307, 1324–1328 (2005).
Craig, A.M. & Kang, Y. Neurexin-neuroligin signaling in synapse development. Curr. Opin. Neurobiol. 17, 43–52 (2007).
Ulucan, C. et al. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 293, H1662–H1672 (2007).
Enserink, J.M. et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4, 901–906 (2002).
Kang, G. et al. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J. Biol. Chem. 278, 8279–8285 (2003).
York, R.D. et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392, 622–626 (1998).
Vossler, M.R. et al. cAMP activates MAP kinase and Elk-1 through a B-Raf– and Rap1-dependent pathway. Cell 89, 73–82 (1997).
Jamain, S. et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 34, 27–29 (2003).
Li, Y. et al. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J. Biol. Chem. 281, 2506–2514 (2006).
Lüthi, A. et al. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron 24, 389–399 (1999).
Xia, J., Chung, H.J., Wihler, C., Huganir, R.L. & Linden, D.J. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain–containing proteins. Neuron 28, 499–510 (2000).
Cheung, U., Atwood, H.L. & Zucker, R.S. Presynaptic effectors contributing to cAMP-induced synaptic potentiation in Drosophila. J. Neurobiol. 66, 273–280 (2006).
Zhong, N. & Zucker, R.S. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J. Neurosci. 25, 208–214 (2005).
Gekel, I. & Neher, E. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J. Neurosci. 28, 7991–8002 (2008).
Ster, J. et al. Epac mediates PACAP-dependent long-term depression in the hippocampus. J. Physiol. (Lond.) 587, 101–113 (2009).
Gelinas, J.N. et al. Activation of exchange protein activated by cyclic-AMP enhances long-lasting synaptic potentiation in the hippocampus. Learn. Mem. 15, 403–411 (2008).
Ouyang, M., Zhang, L., Zhu, J.J., Schwede, F. & Thomas, S.A. Epac signaling is required for hippocampus-dependent memory retrieval. Proc. Natl. Acad. Sci. USA 105, 11993–11997 (2008).
Kelly, M.P. et al. Developmental etiology for neuroanatomical and cognitive deficits in mice overexpressing Galphas, a G-protein subunit genetically linked to schizophrenia. Mol. Psychiatry 14, 398–415 (2009).
Frey, U., Huang, Y.Y. & Kandel, E.R. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260, 1661–1664 (1993).
Otmakhov, N. & Lisman, J.E. Postsynaptic application of a cAMP analogue reverses long-term potentiation in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 87, 3018–3032 (2002).
Chen, Z. et al. Roles of dopamine receptors in long-term depression: enhancement via D1 receptors and inhibition via D2 receptors. Receptors Channels 4, 1–8 (1996).
Huang, Y.Y., Simpson, E., Kellendonk, C. & Kandel, E.R. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc. Natl. Acad. Sci. USA 101, 3236–3241 (2004).
Otani, S., Blond, O., Desce, J.M. & Crepel, F. Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neuroscience 85, 669–676 (1998).
Gereau, R.W. IV & Conn, P.J. Potentiation of cAMP responses by metabotropic glutamate receptors depresses excitatory synaptic transmission by a kinase-independent mechanism. Neuron 12, 1121–1129 (1994).
Smith, W.B., Starck, S.R., Roberts, R.W. & Schuman, E.M. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron 45, 765–779 (2005).
Kayser, M.S., Nolt, M.J. & Dalva, M.B. EphB receptors couple dendritic filopodia motility to synapse formation. Neuron 59, 56–69 (2008).
Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Kojima, S., Vignjevic, D. & Borisy, G.G. Improved silencing vector coexpressing GFP and small hairpin RNA. Biotechniques 36, 74–79 (2004).
Dunaevsky, A., Tashiro, A., Majewska, A., Mason, C. & Yuste, R. Developmental regulation of spine motility in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 96, 13438–13443 (1999).
Acknowledgements
We thank R.L. Huganir (Johns Hopkins University) for antibodies to AMPAR and NMDAR subunits, J. Bos (Utrecht University) and P. Stork (Vollum Institute) for plasmids, A. El-Husseini (University of British Columbia) and P. Scheiffele (University of Basel) for antibodies to neuroligin and plasmid constructs, and G. Borisy and S.-I. Kojima (Northwestern University) for the pGSuper plasmid. We thank A.K. Srivastava (J.C. Self Research Institute of Human Genetics) and G. Swanson (Northwestern University) for thoughtful discussion. This work was supported by the National Alliance for Autism Research, the National Alliance for Research on Schizophrenia and Depression, the Alzheimer's Association, grants from the US National Institutes of Health (MH 071316 to P.P., NS057499 to M.P. and CA108647 to L.A.Q.), a pre-doctoral American Heart Association fellowship to K.M.W. and a post-doctoral American Heart Association fellowship to D.P.S.
Author information
Authors and Affiliations
Contributions
K.M.W. and D.P.S. designed and performed the experiments; H.P., M.V.B., M.E.C., Z.X. and K.A.J. carried out experiments; M.Y. and M.P. performed the mEPSC experiments and assisted in data analysis; L.A.Q. contributed reagents and provided technical expertise; and K.M.W., D.P.S. and P.P. wrote the manuscript. P.P. directed the project.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13, Supplementary Tables 1 and 2, Supplementary Discussion and Supplementary Results (PDF 2005 kb)
Rights and permissions
About this article
Cite this article
Woolfrey, K., Srivastava, D., Photowala, H. et al. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci 12, 1275–1284 (2009). https://doi.org/10.1038/nn.2386
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2386
This article is cited by
-
CaMKII: a central molecular organizer of synaptic plasticity, learning and memory
Nature Reviews Neuroscience (2022)
-
The Rap1 small GTPase is a critical mediator of the effects of stress on prefrontal cortical dysfunction
Molecular Psychiatry (2021)
-
Targeting of δ-catenin to postsynaptic sites through interaction with the Shank3 N-terminus
Molecular Autism (2020)
-
The genome-wide risk alleles for psychiatric disorders at 3p21.1 show convergent effects on mRNA expression, cognitive function, and mushroom dendritic spine
Molecular Psychiatry (2020)
-
Comprehensive behavioral analysis of mice deficient in Rapgef2 and Rapgef6, a subfamily of guanine nucleotide exchange factors for Rap small GTPases possessing the Ras/Rap-associating domain
Molecular Brain (2018)