Abstract
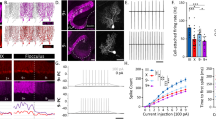
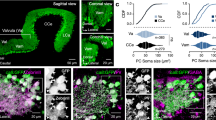
Correlated network activity is important in the development of many neural circuits. Purkinje cells are among the first neurons to populate the cerebellar cortex, where they sprout exuberant axon collaterals. We used multiple patch-clamp recordings targeted with two-photon microscopy to characterize monosynaptic connections between the Purkinje cells of juvenile mice. We found that Purkinje cell axon collaterals projected asymmetrically in the sagittal plane, directed away from the lobule apex. On the basis of our anatomical and physiological characterization of this connection, we constructed a network model that robustly generated waves of activity that traveled along chains of connected Purkinje cells. Consistent with the model, we observed traveling waves of activity in Purkinje cells in sagittal slices from young mice that require GABAA receptor–mediated transmission and intact Purkinje cell axon collaterals. These traveling waves are absent in adult mice, suggesting they have a developmental role in wiring the cerebellar cortical microcircuit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ivry, R. Cerebellar timing systems. Int. Rev. Neurobiol. 41, 555–573 (1997).
Chan-Palay, V. The recurrent collaterals of Purkinje cell axons: a correlated study of the rat's cerebellar cortex with electron microscopy and the Golgi method. Z. Anat. Entwicklungsgesch. 134, 200–234 (1971).
Ramon y Cajal, S. Histologie du Systeme Nerveux de l'homme et des Vertebres (Maloine, Paris, 1911).
Larramendi, L.M. & Lemkey-Johnston, N. The distribution of recurrent Purkinje collateral synapses in the mouse cerebellar cortex: an electron microscopic study. J. Comp. Neurol. 138, 451–459 (1970).
Hamori, J. & Szentagothai, J. Identification of synapses formed in the cerebellar cortex by Purkinje axon collaterals: an electron microscope study. Exp. Brain Res. 5, 118–128 (1968).
De Camilli, P., Miller, P.E., Levitt, P., Walter, U. & Greengard, P. Anatomy of cerebellar Purkinje cells in the rat determined by a specific immunohistochemical marker. Neuroscience 11, 761–817 (1984).
Orduz, D. & Llano, I. Recurrent axon collaterals underlie facilitating synapses between cerebellar Purkinje cells. Proc. Natl. Acad. Sci. USA 104, 17831–17836 (2007).
Maex, R. & De Schutter, E. Oscillations in the cerebellar cortex: a prediction of their frequency bands. Prog. Brain Res. 148, 181–188 (2005).
Sotelo, C. Cellular and genetic regulation of the development of the cerebellar system. Prog. Neurobiol. 72, 295–339 (2004).
Gianola, S., Savio, T., Schwab, M.E. & Rossi, F. Cell-autonomous mechanisms and myelin-associated factors contribute to the development of Purkinje axon intracortical plexus in the rat cerebellum. J. Neurosci. 23, 4613–4624 (2003).
Altman, J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J. Comp. Neurol. 145, 399–463 (1972).
Feller, M.B. Spontaneous correlated activity in developing neural circuits. Neuron 22, 653–656 (1999).
Ben-Ari, Y. Developing networks play a similar melody. Trends Neurosci. 24, 353–360 (2001).
Katz, L.C. & Shatz, C.J. Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996).
Sekirnjak, C., Vissel, B., Bollinger, J., Faulstich, M. & du Lac, S. Purkinje cell synapses target physiologically unique brainstem neurons. J. Neurosci. 23, 6392–6398 (2003).
Bali, B., Erdélyi, F., Szabó, G. & Kovacs, K.J. Visualization of stress-responsive inhibitory circuits in the GAD65-eGFP transgenic mice. Neurosci. Lett. 380, 60–65 (2005).
Erdélyi, F. et al. GAD65-GFP transgenic mice expressing GFP in the GABAergic nervous system. FENS Abstr. 1, A011.3 (2003).
Raman, I.M. & Bean, B.P. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J. Neurosci. 17, 4517–4526 (1997).
Häusser, M. & Clark, B.A. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron 19, 665–678 (1997).
Mittmann, W. & Häusser, M. Linking synaptic plasticity and spike output at excitatory and inhibitory synapses onto cerebellar Purkinje cells. J. Neurosci. 27, 5559–5570 (2007).
Eilers, J., Plant, T.D., Marandi, N. & Konnerth, A. GABA-mediated Ca2+ signaling in developing rat cerebellar Purkinje neurones. J. Physiol. (Lond.) 536, 429–437 (2001).
Vida, I., Bartos, M. & Jonas, P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron 49, 107–117 (2006).
Khaliq, Z.M., Gouwens, N.W. & Raman, I.M. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J. Neurosci. 23, 4899–4912 (2003).
Firth, S.I., Wang, C.T. & Feller, M.B. Retinal waves: mechanisms and function in visual system development. Cell Calcium 37, 425–432 (2005).
Yanik, M.F. et al. Neurosurgery: functional regeneration after laser axotomy. Nature 432, 822 (2004).
Mejia-Gervacio, S. et al. Axonal speeding: shaping synaptic potentials in small neurons by the axonal membrane compartment. Neuron 53, 843–855 (2007).
O'Donoghue, D.L., King, J.S. & Bishop, G.A. Physiological and anatomical studies of the interactions between Purkinje cells and basket cells in the cat's cerebellar cortex: evidence for a unitary relationship. J. Neurosci. 9, 2141–2150 (1989).
Brody, C.D. Correlations without synchrony. Neural Comput. 11, 1537–1551 (1999).
de la Rocha, J., Doiron, B., Shea-Brown, E., Josic, K. & Reyes, A. Correlation between neural spike trains increases with firing rate. Nature 448, 802–806 (2007).
Hawkes, R. & Leclerc, N. Purkinje cell axon collateral distributions reflect the chemical compartmentation of the rat cerebellar cortex. Brain Res. 476, 279–290 (1989).
Song, S., Sjöstrom, P.J., Reigl, M., Nelson, S. & Chklovskii, D.B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, e68 (2005).
Pedroarena, C.M. & Schwarz, C. Efficacy and short-term plasticity at GABAergic synapses between Purkinje and cerebellar nuclei neurons. J. Neurophysiol. 89, 704–715 (2003).
Telgkamp, P. & Raman, I.M. Depression of inhibitory synaptic transmission between Purkinje cells and neurons of the cerebellar nuclei. J. Neurosci. 22, 8447–8457 (2002).
Midtgaard, J. Stellate cell inhibition of Purkinje cells in the turtle cerebellum in vitro. J. Physiol. (Lond.) 457, 355–367 (1992).
Pouzat, C. & Hestrin, S. Developmental regulation of basket/stellate cell → Purkinje cell synapses in the cerebellum. J. Neurosci. 17, 9104–9112 (1997).
Reyes, A. & Sakmann, B. Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. J. Neurosci. 19, 3827–3835 (1999).
Ben-Ari, Y. Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739 (2002).
Douglas, R.J. & Martin, K.A. Recurrent neuronal circuits in the neocortex. Curr. Biol. 17, R496–R500 (2007).
Connors, B.W. & Telfeian, A.E. Dynamic properties of cells, synapses, circuits and seizures in neocortex. Adv. Neurol. 84, 141–152 (2000).
Cohen, A.H. et al. Modelling of intersegmental coordination in the lamprey central pattern generator for locomotion. Trends Neurosci. 15, 434–438 (1992).
de Solages, C. et al. High-frequency organization and synchrony of activity in the purkinje cell layer of the cerebellum. Neuron 58, 775–788 (2008).
Geisler, C., Brunel, N. & Wang, X.J. Contributions of intrinsic membrane dynamics to fast network oscillations with irregular neuronal discharges. J. Neurophysiol. 94, 4344–4361 (2005).
Lee, S.H., Blake, R. & Heeger, D.J. Traveling waves of activity in primary visual cortex during binocular rivalry. Nat. Neurosci. 8, 22–23 (2005).
De Zeeuw, C.I., Hoebeek, F.E. & Schonewille, M. Causes and consequences of oscillations in the cerebellar cortex. Neuron 58, 655–658 (2008).
Young, J.M. et al. Cortical reorganization consistent with spike timing, but not correlation, dependent plasticity. Nat. Neurosci. 10, 887–895 (2007).
Kerschensteiner, D. & Wong, R.O. A precisely timed asynchronous pattern of ON and OFF retinal ganglion cell activity during propagation of retinal waves. Neuron 58, 851–858 (2008).
Braitenberg, V. Functional interpretation of cerebellar histology. Nature 190, 539–540 (1961).
Eccles, J.C., Szentagothai, J. & Ito, M. The Cerebellum as a Neuronal Machine (Springer-Verlag, Heidelberg, 1967).
Oberdick, J., Baader, S.L. & Schilling, K. From zebra stripes to postal zones: deciphering patterns of gene expression in the cerebellum. Trends Neurosci. 21, 383–390 (1998).
Rall, W. Theoretical significance of dendritic trees for neuronal input-output relations. in Neural Theory and Modeling (ed. Reiss, R.F. 73–97 (Stanford University Press, Stanford, California, USA, 1964).
Acknowledgements
We thank B. Clark, I. Duguid, F. Edwards, S. Ho, T. Ishikawa, M. London, E. Rancz, A. Roth and S. Smith for helpful discussions and for comments on the manuscript. We are grateful to S. du Lac, G. Szabó and F. Erdélyi for providing transgenic mice, to J. Gruendemann for providing tissue for reconstructions, to B. Clark for help with perfusions, and to L. Ramakrishnan and K. Powell for expert assistance with histology and Neurolucida reconstructions. This work was funded by a European Molecular Biology Organization Long-Term Fellowship and a Royal Society Dorothy Hodgkin Fellowship to A.J.W., a Feodor Lynen Fellowship of the Alexander von Humboldt Foundation to H.C., a European Young Investigator Award and a Wellcome Trust project grant to Z.N., a Marie-Curie Intra-European fellowship and Medical Research Council Career Development Award to P.J.S., and a Wellcome Trust Senior Research Fellowship and a grant from the Gatsby Foundation to M.H.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 – 7, Supplementary Table 1 and Supplementary Methods (PDF 6080 kb)
Supplementary Movie 1
Rotation of superimposed reconstructed Purkinje cells (n = 39) showing that axon collaterals lie predominantly in the sagittal plane (see Fig. 3b for scale bar). (MOV 2645 kb)
Supplementary Movie 2
Visualization of traveling waves in a model network of Purkinje cells connected by depolarizing synapses (Erev = −40 mV; see Fig. 5b). Cell location in the lobule is represented acoustically (low pitch = apex, high pitch = base of lobule). (MOV 2547 kb)
Supplementary Movie 3
Visualization of traveling waves in a model network of Purkinje cells connected by hyperpolarizing synapses (Erev = −80 mV; see Fig. 5c). Cell location in the lobule is represented acoustically (low pitch = apex, high pitch = base of lobule). (MOV 2561 kb)
Rights and permissions
About this article
Cite this article
Watt, A., Cuntz, H., Mori, M. et al. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci 12, 463–473 (2009). https://doi.org/10.1038/nn.2285
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2285
This article is cited by
-
Purkinje cell axonal swellings enhance action potential fidelity and cerebellar function
Nature Communications (2021)
-
Emerging connections between cerebellar development, behaviour and complex brain disorders
Nature Reviews Neuroscience (2019)
-
Molecular layer interneurons shape the spike activity of cerebellar Purkinje cells
Scientific Reports (2019)
-
ATAT1 regulates forebrain development and stress-induced tubulin hyperacetylation
Cellular and Molecular Life Sciences (2019)