Abstract
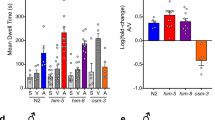
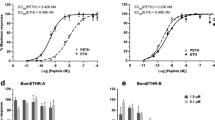
Egg-laying behavior of the Caenorhabditis elegans hermaphrodite is regulated by G protein signaling pathways. Here we show that the egg laying–defective mutant egl-6(n592) carries an activating mutation in a G protein–coupled receptor that inhibits C. elegans egg-laying motor neurons in a Go-dependent manner. Ligands for EGL-6 are Phe-Met-Arg-Phe-NH2 (FMRFamide)-related peptides encoded by the genes flp-10 and flp-17. flp-10 is expressed in both neurons and non-neuronal cells. The major source of flp-17 peptides is a pair of presumptive sensory neurons, the BAG neurons. Genetic analysis of the egl-6 pathway revealed that the EGL-6 neuropeptide signaling pathway functions redundantly with acetylcholine to inhibit egg-laying. The retention of embryos in the uterus of the C. elegans hermaphrodite is therefore under the control of a presumptive sensory system and is inhibited by the convergence of signals from neuropeptides and the small-molecule neurotransmitter acetylcholine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Horvitz, H.R., Chalfie, M., Trent, C., Sulston, J.E. & Evans, P.D. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216, 1012–1014 (1982).
Trent, C., Tsung, N. & Horvitz, H.R. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104, 619–647 (1983).
White, J.G., Southgate, E., Thomson, J.N. & Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B Biol. Sci. 314, 1–340 (1986).
Rand, J.B. & Nonet, M.L. Neurotransmitter assignments for specific neurons. in C. elegans II (eds. Riddle, D.L., Blumenthal, T., Meyer, B.J. & Priess, J.R.) 1049–1052 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1997).
Duerr, J.S., Gaskin, J. & Rand, J.B. Identified neurons in C. elegans coexpress vesicular transporters for acetylcholine and monoamines. Am. J. Physiol. Cell Physiol. 280, C1616–C1622 (2001).
Waggoner, L.E., Zhou, G.T., Schafer, R.W. & Schafer, W.R. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron 21, 203–214 (1998).
Bany, I.A., Dong, M.Q. & Koelle, M.R. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J. Neurosci. 23, 8060–8069 (2003).
Mendel, J.E. et al. Participation of the protein Go in multiple aspects of behavior in C. elegans. Science 267, 1652–1655 (1995).
Segalat, L., Elkes, D.A. & Kaplan, J.M. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science 267, 1648–1651 (1995).
Brundage, L. et al. Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron 16, 999–1009 (1996).
Lackner, M.R., Nurrish, S.J. & Kaplan, J.M. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24, 335–346 (1999).
Miller, K.G., Emerson, M.D. & Rand, J.B. Goα and diacylglycerol kinase negatively regulate the Gqα pathway in C. elegans. Neuron 24, 323–333 (1999).
Nurrish, S., Segalat, L. & Kaplan, J.M. Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron 24, 231–242 (1999).
Carnell, L., Illi, J., Hong, S.W. & McIntire, S.L. The G-protein-coupled serotonin receptor SER-1 regulates egg laying and male mating behaviors in Caenorhabditis elegans. J. Neurosci. 25, 10671–10681 (2005).
Hobson, R.J. et al. SER-7, a Caenorhabditis elegans 5-HT7-like receptor, is essential for the 5-HT stimulation of pharyngeal pumping and egg laying. Genetics 172, 159–169 (2006).
Shyn, S.I., Kerr, R. & Schafer, W.R. Serotonin and Go modulate functional states of neurons and muscles controlling C. elegans egg-laying behavior. Curr. Biol. 13, 1910–1915 (2003).
Kass, J., Jacob, T.J., Kim, P. & Kaplan, J.M. The EGL-3 prohormone convertase regulates mechanosensory responses of Caenorhabditis elegans. J. Neurosci. 21, 9265–9272 (2001).
Jacob, T.C. & Kaplan, J.M. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J. Neurosci. 23, 2122–2130 (2003).
Moresco, J.J. & Koelle, M.R. Activation of EGL-47, a Galpha(o)-coupled receptor, inhibits function of hermaphrodite-specific motor neurons to regulate Caenorhabditis elegans egg-laying behavior. J. Neurosci. 24, 8522–8530 (2004).
Gether, U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 21, 90–113 (2000).
Sze, J.Y., Victor, M., Loer, C., Shi, Y. & Ruvkun, G. Food and metabolic signaling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403, 560–564 (2000).
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Cazzamali, G. & Grimmelikhuijzen, C.J. Molecular cloning and functional expression of the first insect FMRFamide receptor. Proc. Natl. Acad. Sci. USA 99, 12073–12078 (2002).
Meeusen, T. et al. Identification in Drosophila melanogaster of the invertebrate G protein-coupled FMRFamide receptor. Proc. Natl. Acad. Sci. USA 99, 15363–15368 (2002).
Li, C. The ever-expanding neuropeptide gene families in the nematode Caenorhabditis elegans. Parasitology 131 Suppl: S109–S127 (2005).
Nathoo, A.N., Moeller, R.A., Westlund, B.A. & Hart, A.C. Identification of neuropeptide-like gene families in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 98, 14000–14005 (2001).
Rogers, C. et al. Inhibition of Caenorhabditis elegans social feeding by FMRFamide-related peptide activation of NPR-1. Nat. Neurosci. 6, 1178–1185 (2003).
Kim, K. & Li, C. Expression and regulation of an FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. J. Comp. Neurol. 475, 540–550 (2004).
Jin, Y., Jorgensen, E., Hartwieg, E. & Horvitz, H.R. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 19, 539–548 (1999).
Sulston, J., Dew, M. & Brenner, S. Dopaminergic neurons in the nematode C. elegans. J. Comp. Neurol. 163, 215–226 (1975).
Lee, R.Y., Sawin, E.R., Chalfie, M., Horvitz, H.R. & Avery, L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J. Neurosci. 19, 159–167 (1999).
Alkema, M.J., Hunter-Ensor, M., Ringstad, N. & Horvitz, H.R. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46, 247–260 (2005).
Alfonso, A., Grundahl, K., Duerr, J.S., Han, H.P. & Rand, J.B. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science 261, 617–619 (1993).
Alfonso, A., Grundahl, K., McManus, J.R. & Rand, J.B. Cloning and characterization of the choline acetyltransferase structural gene (cha-1) from C. elegans. J. Neurosci. 14, 2290–2300 (1994).
Rand, J.B. Genetic analysis of the cha-1-unc-17 gene complex in Caenorhabditis. Genetics 122, 73–80 (1989).
Culotti, J.G., Von Ehrenstein, G., Culotti, M.R. & Russell, R.L. A second class of acetylcholinesterase-deficient mutants of the nematode Caenorhabditis elegans. Genetics 97, 281–305 (1981).
Ward, S., Thomson, N., White, J.G. & Brenner, S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 160, 313–337 (1975).
Ware, R.W., Clark, D., Crossland, K. & Russell, R.L. The nerve ring of the nematode Caenorhabditis elegans: sensory input and motor output. J. Comp. Neurol. 162, 71–110 (1975).
Jose, A.M., Bany, I.A., Chase, D.L. & Koelle, M.R. A specific subset of transient receptor potential vanilloid-type channel subunits in Caenorhabditis elegans endocrine cells function as mixed heteromers to promote neurotransmitter release. Genetics 175, 93–105 (2007).
Lee, Y.S. et al. Characterization of GAR-2, a novel G protein-linked acetylcholine receptor from Caenorhabditis elegans. J. Neurochem. 75, 1800–1809 (2000).
Hwang, J.M. et al. Cloning and functional characterization of a Caenorhabditis elegans muscarinic acetylcholine receptor. Receptors Channels 6, 415–424 (1999).
Lee, Y.S. et al. Cloning and expression of a G protein-linked acetylcholine receptor from Caenorhabditis elegans. J. Neurochem. 72, 58–65 (1999).
Putrenko, I., Zakikhani, M. & Dent, J.A. A family of acetylcholine-gated chloride channel subunits in Caenorhabditis elegans. J. Biol. Chem. 280, 6392–6398 (2005).
Weinshenker, D., Garriga, G. & Thomas, J.H. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J. Neurosci. 15, 6975–6985 (1995).
Hallem, E.A. & Sternberg, P.W. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 105, 8038–8043 (2008).
Wicks, S.R., Yeh, R.T., Gish, W.R., Waterston, R.H. & Plasterk, R.H. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28, 160–164 (2001).
Jansen, G., Hazendonk, E., Thijssen, K.L. & Plasterk, R.H. Reverse genetics by chemical mutagenesis in Caenorhabditis elegans. Nat. Genet. 17, 119–121 (1997).
Finney, M. & Ruvkun, G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell 63, 895–905 (1990).
Liman, E.R., Tytgat, J. & Hess, P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9, 861–871 (1992).
R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051–07–0, URL http://www.R-project.org (2006).
Bargmann, C.I. & Avery, L. Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol. 48, 225–250 (1995).
Acknowledgements
We thank Y. Kohara for flp-10 and flp-17 cDNAs; N. Dascal for GIRK1 and GIRK4 expression constructs; J. Rand for unc-17 cha-1 strains; J. Moresco and M. Koelle for tph-1 promoter constructs; A. Fire for expression vectors; A. Hellman, S. McGonagle, B. Castor and N. An for technical assistance; N. Abe and R. O'Hagan for help with Xenopus oocyte electrophysiology; J. Chung for help with data analysis; and B. Galvin and A. Saffer for critical reading of the manuscript. N.R. received support from the Howard Hughes Medical Institute, the Life Sciences Research Foundation and the Medical Foundation. H.R.H. is a David H. Koch Professor of Biology and an Investigator of the Howard Hughes Medical Institute. This work was supported by National Institutes of Health grant GM24663.
Author information
Authors and Affiliations
Contributions
N.R. performed all experiments. N.R. and H.R.H. designed the experiments and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1, Supplementary Tables 1 and 2 and Supplementary Methods (PDF 274 kb)
Rights and permissions
About this article
Cite this article
Ringstad, N., Horvitz, H. FMRFamide neuropeptides and acetylcholine synergistically inhibit egg-laying by C. elegans. Nat Neurosci 11, 1168–1176 (2008). https://doi.org/10.1038/nn.2186
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2186
This article is cited by
-
Multisite regulation integrates multimodal context in sensory circuits to control persistent behavioral states in C. elegans
Nature Communications (2023)
-
Distinct neuropeptide-receptor modules regulate a sex-specific behavioral response to a pheromone
Communications Biology (2021)
-
BTBD9 and dopaminergic dysfunction in the pathogenesis of restless legs syndrome
Brain Structure and Function (2020)
-
Systematic evaluation of C. elegans lincRNAs with CRISPR knockout mutants
Genome Biology (2019)
-
Control of Neuropeptide Expression by Parallel Activity-dependent Pathways in Caenorhabditis elegans
Scientific Reports (2017)