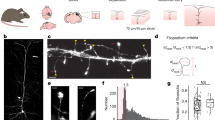
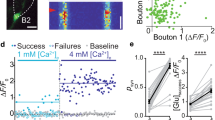
Abstract
Synaptogenesis and the role of dendritic protrusions in this process are well studied in glutamatergic synapses. Much less is known about the formation of GABAergic synapses, which are located predominantly on the dendritic shaft. We used genetically labeled interneurons in mature hippocampal slice cultures and two-photon laser-scanning microscopy to examine contact formation between GABAergic axons and the dendrites of CA1 pyramidal cells. Dendritic protrusions distinguished and selected between glutamatergic and GABAergic boutons. In contrast with contacts with glutamatergic boutons, which can be long lasting, the contacts of dendritic protrusions with GABAergic boutons were always short lived. Similarly, the contacts made by GABAergic axonal protrusions were always transient. New putative GABAergic synapses were formed exclusively by new boutons appearing at pre-existing axon-dendrite crossings without the involvement of any dendritic or axonal protrusions. These findings imply that fundamentally different mechanisms underlie the generation of GABAergic and glutamatergic synapses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Freund, T.F. & Buzsaki, G. Interneurons of the hippocampus. Hippocampus 6, 347–470 (1996).
Harris, K.M. & Kater, S.B. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 17, 341–371 (1994).
Megías, M., Emri, Z., Freund, T.F. & Gulyás, A.I. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102, 527–540 (2001).
Ziv, N.E. & Smith, S.J. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17, 91–102 (1996).
Fiala, J.C., Feinberg, M., Popov, V. & Harris, K.M. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J. Neurosci. 18, 8900–8911 (1998).
Petrak, L.J., Harris, K.M. & Kirov, S.A. Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission. J. Comp. Neurol. 484, 183–190 (2005).
Knott, G.W., Holtmaat, A., Wilbrecht, L., Welker, E. & Svoboda, K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat. Neurosci. 9, 1117–1124 (2006).
Nägerl, U.V., Kostinger, G., Anderson, J.C., Martin, K.A. & Bonhoeffer, T. Protracted synaptogenesis after activity-dependent spinogenesis in hippocampal neurons. J. Neurosci. 27, 8149–8156 (2007).
De Roo, M., Klauser, P., Mendez, P., Poglia, L. & Muller, D. Activity-dependent PSD formation and stabilization of newly formed spines in hippocampal slice cultures. Cereb. Cortex 18, 151–161 (2008).
Lohmann, C., Finski, A. & Bonhoeffer, T. Local calcium transients regulate the spontaneous motility of dendritic filopodia. Nat. Neurosci. 8, 305–312 (2005).
Lohmann, C. & Bonhoeffer, T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron 59, 253–260 (2008).
Dailey, M.E. & Smith, S.J. The dynamics of dendritic structure in developing hippocampal slices. J. Neurosci. 16, 2983–2994 (1996).
Marrs, G.S., Green, S.H. & Dailey, M.E. Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nat. Neurosci. 4, 1006–1013 (2001).
Saito, Y., Song, W.J. & Murakami, F. Preferential termination of corticorubral axons on spine-like dendritic protrusions in developing cat. J. Neurosci. 17, 8792–8803 (1997).
Yuste, R. & Bonhoeffer, T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat. Rev. Neurosci. 5, 24–34 (2004).
Somogyi, P., Tamás, G., Lujan, R. & Buhl, E.H. Salient features of synaptic organization in the cerebral cortex. Brain Res. Brain Res. Rev. 26, 113–135 (1998).
López-Bendito, G. et al. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb. Cortex 14, 1122–1133 (2004).
Kalisman, N., Silberberg, G. & Markram, H. The neocortical microcircuit as a tabula rasa. Proc. Natl. Acad. Sci. USA 102, 880–885 (2005).
Shepherd, G.M. & Harris, K.M. Three-dimensional structure and composition of CA3 → CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. 18, 8300–8310 (1998).
Meyer, M.P. & Smith, S.J. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J. Neurosci. 26, 3604–3614 (2006).
De Paola, V. et al. Cell type–specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49, 861–875 (2006).
Ruthazer, E.S., Li, J. & Cline, H.T. Stabilization of axon branch dynamics by synaptic maturation. J. Neurosci. 26, 3594–3603 (2006).
Ahmari, S.E., Buchanan, J. & Smith, S.J. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 3, 445–451 (2000).
Friedman, H.V., Bresler, T., Garner, C.C. & Ziv, N.E. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron 27, 57–69 (2000).
Bresler, T. et al. Postsynaptic density assembly is fundamentally different from presynaptic active zone assembly. J. Neurosci. 24, 1507–1520 (2004).
Kubota, Y., Hatada, S., Kondo, S., Karube, F. & Kawaguchi, Y. Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J. Neurosci. 27, 1139–1150 (2007).
Knott, G.W., Quairiaux, C., Genoud, C. & Welker, E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 34, 265–273 (2002).
Risher, W.C., Ostroff, L.E. & Harris, K.M. What dendritic filopodia induced by LTP encounter along their path through the neuropil of PN15 rat hippocampus. Abstr. Soc. Neurosci. 135.4 (2006).
Ziv, N.E. & Garner, C.C. Cellular and molecular mechanisms of presynaptic assembly. Nat. Rev. Neurosci. 5, 385–399 (2004).
Gerrow, K. et al. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron 49, 547–562 (2006).
Maas, C. et al. Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. J. Cell Biol. 172, 441–451 (2006).
Chattopadhyaya, B. et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron 54, 889–903 (2007).
Di Cristo, G. et al. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci. 10, 1569–1577 (2007).
Ahmari, S.E. & Smith, S.J. Knowing a nascent synapse when you see it. Neuron 34, 333–336 (2002).
Portera-Cailliau, C., Pan, D.T. & Yuste, R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J. Neurosci. 23, 7129–7142 (2003).
Richards, D.A. et al. Glutamate induces the rapid formation of spine head protrusions in hippocampal slice cultures. Proc. Natl. Acad. Sci. USA 102, 6166–6171 (2005).
de Zeeuw, C.I., Ruigrok, T.J., Holstege, J.C., Jansen, H.G. & Voogd, J. Intracellular labeling of neurons in the medial accessory olive of the cat. II. Ultrastructure of dendritic spines and their GABAergic innervation. J. Comp. Neurol. 300, 478–494 (1990).
Cope, D.W. et al. Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurones target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience 109, 63–80 (2002).
Pratt, K.G., Taft, C.E., Burbea, M. & Turrigiano, G.G. Dynamics underlying synaptic gain between pairs of cortical pyramidal neurons. Dev. Neurobiol. 68, 143–151 (2008).
Sabo, S.L., Gomes, R.A. & McAllister, A.K. Formation of presynaptic terminals at predefined sites along axons. J. Neurosci. 26, 10813–10825 (2006).
Graf, E.R., Zhang, X., Jin, S.X., Linhoff, M.W. & Craig, A.M. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119, 1013–1026 (2004).
Chih, B., Engelman, H. & Scheiffele, P. Control of excitatory and inhibitory synapse formation by neuroligins. Science 307, 1324–1328 (2005).
Chubykin, A.A. et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54, 919–931 (2007).
Stepanyants, A., Tamás, G. & Chklovskii, D.B. Class-specific features of neuronal wiring. Neuron 43, 251–259 (2004).
Thomson, A.M. & Morris, O.T. Selectivity in the inter-laminar connections made by neocortical neurones. J. Neurocytol. 31, 239–246 (2002).
Karube, F., Kubota, Y. & Kawaguchi, Y. Axon branching and synaptic bouton phenotypes in GABAergic nonpyramidal cell subtypes. J. Neurosci. 24, 2853–2865 (2004).
Shepherd, G.M., Raastad, M. & Andersen, P. General and variable features of varicosity spacing along unmyelinated axons in the hippocampus and cerebellum. Proc. Natl. Acad. Sci. USA 99, 6340–6345 (2002).
Gähwiler, B.H. Organotypic monolayer cultures of nervous tissue. J. Neurosci. Methods 4, 329–342 (1981).
Acknowledgements
We would like to thank G. Szábo for kindly providing the GAD65-GFP mice, U.V. Nägerl for help with the experimental setup and comments on the manuscript, N. Stöhr and C. Huber for technical assistance, and T. Mrsic-Flögel, C. Lohmann and V. Stein for critical reading of the manuscript. This work was supported by the Max Planck Gesellschaft, the Alexander von Humboldt Stiftung, a Marie Curie Intra-European fellowship (C.J.W.) and the Boehringer Ingelheim Fonds (N.B.).
Author information
Authors and Affiliations
Contributions
C.J.W. designed and conducted the experiments and analyzed the data. N.B. carried out the experiments on glutamatergic boutons. C.J.W. and T.B. conceived the project and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 725 kb)
Rights and permissions
About this article
Cite this article
Wierenga, C., Becker, N. & Bonhoeffer, T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat Neurosci 11, 1044–1052 (2008). https://doi.org/10.1038/nn.2180
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2180
This article is cited by
-
Synaptic input and Ca2+ activity in zebrafish oligodendrocyte precursor cells contribute to myelin sheath formation
Nature Neuroscience (2024)
-
GSK-3β orchestrates the inhibitory innervation of adult-born dentate granule cells in vivo
Cellular and Molecular Life Sciences (2023)
-
Cocaine increases dopaminergic connectivity in the nucleus accumbens
Brain Structure and Function (2018)
-
Structure, Distribution, and Function of Neuronal/Synaptic Spinules and Related Invaginating Projections
NeuroMolecular Medicine (2015)
-
Studying synaptic efficiency by post-hoc immunolabelling
BMC Neuroscience (2013)