Abstract
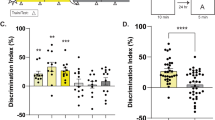
The mitogen-activated protein kinase (MAPK) and cyclic adenosine monophosphate (cAMP) signal transduction pathways have critical roles in the consolidation of hippocampus-dependent memory. We found that extracellular regulated kinase 1/2 MAPK phosphorylation and cAMP underwent a circadian oscillation in the hippocampus that was paralleled by changes in Ras activity and the phosphorylation of MAPK kinase and cAMP response element–binding protein (CREB). The nadir of this activation cycle corresponded with severe deficits in hippocampus-dependent fear conditioning under both light-dark and free-running conditions. Circadian oscillations in cAMP and MAPK activity were absent in memory-deficient transgenic mice that lacked Ca2+-stimulated adenylyl cyclases. Furthermore, physiological and pharmacological interference with oscillations in MAPK phosphorylation after the cellular memory consolidation period impaired the persistence of hippocampus-dependent memory. These data suggest that the persistence of long-term memories may depend on reactivation of the cAMP/MAPK/CREB transcriptional pathway in the hippocampus during the circadian cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Impey, S., Obrietan, K. & Storm, D.R. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron 23, 11–14 (1999).
Sweatt, J.D. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 14, 311–317 (2004).
Wang, H. & Storm, D.R. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol. Pharmacol. 63, 463–468 (2003).
Atkins, C.M., Selcher, J.C., Petraitis, J.J., Trzaskos, J.M. & Sweatt, J.D. The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1, 602–609 (1998).
Schafe, G.E. et al. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J. Neurosci. 20, 8177–8187 (2000).
Athos, J., Impey, S., Pineda, V.V., Chen, X. & Storm, D.R. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat. Neurosci. 5, 1119–1120 (2002).
Duvarci, S., Nader, K. & LeDoux, J.E. Activation of extracellular signal–regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur. J. Neurosci. 21, 283–289 (2005).
Kelly, A., Laroche, S. & Davis, S. Activation of mitogen-activated protein kinase/extracellular signal–regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 23, 5354–5360 (2003).
Silva, A.J., Kogan, J.H., Frankland, P.W. & Kida, S. CREB and memory. Annu. Rev. Neurosci. 21, 127–148 (1998).
Ferguson, G.D. & Storm, D.R. Why calcium-stimulated adenylyl cyclases? Physiology (Bethesda) 19, 271–276 (2004).
Scott, R., Bourtchuladze, R., Gossweiler, S., Dubnau, J. & Tully, T. CREB and the discovery of cognitive enhancers. J. Mol. Neurosci. 19, 171–177 (2002).
Kelleher, R.J. III, Govindarajan, A., Jung, H.Y., Kang, H. & Tonegawa, S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116, 467–479 (2004).
Bernabeu, R. et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. USA 94, 7041–7046 (1997).
Wu, Z.L. et al. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc. Natl. Acad. Sci. USA 92, 220–224 (1995).
Wong, S.T. et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron 23, 787–798 (1999).
Sindreu, C.B., Scheiner, Z.S. & Storm, D.R. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron 53, 79–89 (2007).
Huang, Y.Y. et al. A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell 83, 1211–1222 (1995).
Brandon, E.P. et al. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RI beta subunit of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 92, 8851–8855 (1995).
Abel, T. et al. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88, 615–626 (1997).
Cui, Z. et al. Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron 41, 781–793 (2004).
Bekinschtein, P. et al. Persistence of long-term memory storage requires a late protein synthesis– and BDNF-dependent phase in the hippocampus. Neuron 53, 261–277 (2007).
Lee, A.K. & Wilson, M.A. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194 (2002).
Graves, L.A., Heller, E.A., Pack, A.I. & Abel, T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn. Mem. 10, 168–176 (2003).
Gais, S., Plihal, W., Wagner, U. & Born, J. Early sleep triggers memory for early visual discrimination skills. Nat. Neurosci. 3, 1335–1339 (2000).
Huber, R., Ghilardi, M.F., Massimini, M. & Tononi, G. Local sleep and learning. Nature 430, 78–81 (2004).
Devan, B.D. et al. Circadian phase-shifted rats show normal acquisition, but impaired long-term retention of place information in the water task. Neurobiol. Learn. Mem. 75, 51–62 (2001).
Tapp, W.N. & Holloway, F.A. Phase shifting circadian rhythms produces retrograde amnesia. Science 211, 1056–1058 (1981).
Stephan, F.K. & Kovacevic, N.S. Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions. Behav. Biol. 22, 456–462 (1978).
Fernandez, R.I., Lyons, L.C., Levenson, J., Khabour, O. & Eskin, A. Circadian modulation of long-term sensitization in Aplysia. Proc. Natl. Acad. Sci. USA 100, 14415–14420 (2003).
Leirer, V.O., Tanke, E.D. & Morrow, D.G. Time of day and naturalistic prospective memory. Exp. Aging Res. 20, 127–134 (1994).
Maury, P. & Queinnec, Y. Influence of time of 24-hour day on depth of processing in recall memory. Br. J. Psychol. 83, 249–260 (1992).
Folkard, S., Wever, R.A. & Wildgruber, C.M. Multi-oscillatory control of circadian rhythms in human performance. Nature 305, 223–226 (1983).
Chaudhury, D. & Colwell, C.S. Circadian modulation of learning and memory in fear-conditioned mice. Behav. Brain Res. 133, 95–108 (2002).
Hauber, W. & Bareiss, A. Facilitative effects of an adenosine A1/A2 receptor blockade on spatial memory performance of rats: selective enhancement of reference memory retention during the light period. Behav. Brain Res. 118, 43–52 (2001).
Holloway, F.A. & Wansley, R.A. Multiple retention deficits at periodic intervals after active and passive avoidance learning. Behav. Biol. 9, 1–14 (1973).
Obrietan, K., Impey, S. & Storm, D.R. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat. Neurosci. 1, 693–700 (1998).
Selcher, J.C., Atkins, C.M., Trzaskos, J.M., Paylor, R. & Sweatt, J.D. A necessity for MAP kinase activation in mammalian spatial learning. Learn. Mem. 6, 478–490 (1999).
Shalin, S.C. et al. Neuronal MEK is important for normal fear conditioning in mice. J. Neurosci. Res. 75, 760–770 (2004).
Valentinuzzi, V.S. et al. Locomotor response to an open field during C57BL/6J active and inactive phases: differences dependent on conditions of illumination. Physiol. Behav. 69, 269–275 (2000).
Granados-Fuentes, D., Prolo, L.M., Abraham, U. & Herzog, E.D. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J. Neurosci. 24, 615–619 (2004).
Ohta, H., Yamazaki, S. & McMahon, D.G. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 8, 267–269 (2005).
Aschoff, J. Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z. Tierpsychol. 49, 225–249 (1979).
Keiper, M. et al. Epac- and Ca2+-controlled activation of Ras and extracellular signal–regulated kinases by Gs-coupled receptors. J. Biol. Chem. 279, 46497–46508 (2004).
Sharma, S.K. & Carew, T.J. The roles of MAPK cascades in synaptic plasticity and memory in Aplysia: facilitatory effects and inhibitory constraints. Learn. Mem. 11, 373–378 (2004).
Hetman, M. & Gozdz, A. Role of extracellular signal–regulated kinases 1 and 2 in neuronal survival. Eur. J. Biochem. 271, 2050–2055 (2004).
Shimizu, E., Tang, Y.P., Rampon, C. & Tsien, J.Z. NMDA receptor–dependent synaptic reinforcement as a crucial process for memory consolidation. Science 290, 1170–1174 (2000).
Peigneux, P. et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 44, 535–545 (2004).
Mednick, S., Nakayama, K. & Stickgold, R. Sleep-dependent learning: a nap is as good as a night. Nat. Neurosci. 6, 697–698 (2003).
Marshall, L., Helgadottir, H., Molle, M. & Born, J. Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613 (2006).
Born, J., Rasch, B. & Gais, S. Sleep to remember. Neuroscientist 12, 410–424 (2006).
Acknowledgements
We thank H. de la Iglesia for valuable advice concerning some of the circadian procedures. We also would like to thank several members of the Storm lab for insightful discussions and critical readings of this manuscript. This work was supported by a grant from the US National Institutes of Health (NS 20498), a predoctoral Ruth L. Kirschstein US National Institutes of Health Research Award (1 F31 MH075489-01A1) to K.L.E.-M. and a Korea Research Foundation Grant for Young Scientists to S.H. (KRF-2005-213-C00036).
Author information
Authors and Affiliations
Contributions
K.L.E.-M. designed and carried out the experiments and wrote the manuscript. T.P. performed cannulations and assisted with all infusion experiments as well as readings of the manuscript. S.H. carried out experiments using DKO mice, and H.W. contributed to some of the wild-type circadian experiments. G.C.-K.C. provided instruction for the cyclase assays, and both G.C.-K.C. and Z.S.S. contributed to thorough critiques and revisions of the written manuscript. The principal investigator, D.R.S., allowed the research to be conducted in his laboratory and also helped fund the personnel and experiments necessary for the project.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Methods (PDF 850 kb)
Rights and permissions
About this article
Cite this article
Eckel-Mahan, K., Phan, T., Han, S. et al. Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nat Neurosci 11, 1074–1082 (2008). https://doi.org/10.1038/nn.2174
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2174
This article is cited by
-
The guanine nucleotide exchange factor RapGEF2 is required for ERK-dependent immediate-early gene (Egr1) activation during fear memory formation
Cellular and Molecular Life Sciences (2024)
-
Diurnal variation in declarative memory and the involvement of SCOP in cognitive functions in nonhuman primates
Molecular Brain (2023)
-
The clock gene Per1 may exert diurnal control over hippocampal memory consolidation
Neuropsychopharmacology (2023)
-
Circadian clocks, cognition, and Alzheimer’s disease: synaptic mechanisms, signaling effectors, and chronotherapeutics
Molecular Neurodegeneration (2022)
-
Rapid-acting antidepressants and the circadian clock
Neuropsychopharmacology (2022)