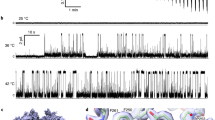
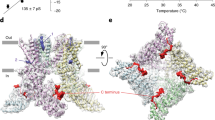
Abstract
Ion channels can be activated (gated) by a variety of stimuli, including chemicals, voltage, mechanical force or temperature. Although molecular mechanisms of ion channel gating by chemical and voltage stimuli are understood in principal, the mechanisms of temperature activation remain unknown. The transient receptor potential channel TRPV3 is a nonselective cation channel that is activated by warm temperatures and sensory chemicals such as camphor. Here we screened ∼14,000 random mutant clones of mouse TRPV3 and identified five single point mutations that specifically abolish heat activation but do not perturb chemical activation or voltage modulation. Notably, all five mutations are located in the putative sixth transmembrane helix and the adjacent extracellular loop in the pore region of mouse TRPV3. Although distinct in sequence, we found that the corresponding loop of frog TRPV3 is also specifically required for heat activation. These findings demonstrate that the temperature sensitivity of TRPV3 is separable from all other known activation mechanisms and implicate a specific region in temperature sensing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Dhaka, A., Viswanath, V. & Patapoutian, A. TRP ion channels and temperature sensation. Annu. Rev. Neurosci. 29, 135–161 (2006).
Caterina, M.J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R64–R76 (2007).
Caterina, M.J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Guler, A.D. et al. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 22, 6408–6414 (2002).
Peier, A.M. et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 296, 2046–2049 (2002).
Xu, H. et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418, 181–186 (2002).
Watanabe, H. et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 277, 13569–13577 (2002).
Smith, G.D. et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418, 186–190 (2002).
Neeper, M.P. et al. Activation properties of heterologously expressed mammalian TRPV2: evidence for species dependence. J. Biol. Chem. 282, 15894–15902 (2007).
McKemy, D.D., Neuhausser, W.M. & Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 (2002).
Peier, A.M. et al. A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 (2002).
Story, G.M. et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 (2003).
Kobayashi, K. et al. Distinct expression of TRPM8, TRPA1 and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 493, 596–606 (2005).
Moqrich, A. et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307, 1468–1472 (2005).
Hu, H.Z. et al. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 279, 35741–35748 (2004).
Vogt-Eisele, A.K. et al. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 151, 530–540 (2007).
Xu, H., Blair, N.T. & Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 25, 8924–8937 (2005).
Owsianik, G., D'Hoedt, D., Voets, T. & Nilius, B. Structure-function relationship of the TRP channel superfamily. Rev. Physiol. Biochem. Pharmacol. 156, 61–90 (2006).
Owsianik, G., Talavera, K., Voets, T. & Nilius, B. Permeation and selectivity of TRP channels. Annu. Rev. Physiol. 68, 685–717 (2006).
Bandell, M., Macpherson, L.J. & Patapoutian, A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr. Opin. Neurobiol. 17, 490–497 (2007).
Nilius, B. et al. Gating of TRP channels: a voltage connection? J. Physiol. (Lond.) 567, 35–44 (2005).
Voets, T. et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430, 748–754 (2004).
Voets, T. et al. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat. Chem. Biol. 3, 174–182 (2007).
Brauchi, S., Orio, P. & Latorre, R. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc. Natl. Acad. Sci. USA 101, 15494–15499 (2004).
Latorre, R. et al. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium 42, 427–438 (2007).
Matta, J.A. & Ahern, G.P. Voltage is a partial activator of thermo-sensitive TRP channels. J. Physiol. 585, 469–482 (2007).
Brauchi, S. et al. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc. Natl. Acad. Sci. USA 104, 10246–10251 (2007).
Brauchi, S. et al. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J. Neurosci. 26, 4835–4840 (2006).
Bandell, M. et al. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat. Neurosci. 9, 493–500 (2006).
Chung, M.K. et al. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J. Neurosci. 24, 5177–5182 (2004).
Chung, M.K., Guler, A.D. & Caterina, M. Biphasic currents evoked by chemical or thermal activation of the heat-gated ion channel, TRPV3. J. Biol. Chem. 280, 15928–15941 (2005).
Long, S.B., Campbell, E.B. & MacKinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005).
Jordt, S.E. et al. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. USA 97, 8134–8139 (2000).
Ryu, S. et al. Uncoupling proton activation of vanilloid receptor TRPV1. J. Neurosci. 27, 12797–12807 (2007).
Myers, B.R., Bohlen, C.J. & Julius, D. A yeast genetic screen reveals a critical role for the pore helix domain in TRP channel gating. Neuron 58, 362–373 (2008).
Filevich, O. & Etchenique, R. 1D and 2D temperature imaging with a fluorescent ruthenium complex. Anal. Chem. 78, 7499–7503 (2006).
Jorgensen, W.L., Chandrasekhar, J., Madura, J.D., Impey, R.W. & Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Acknowledgements
We thank A.J. Wilson and J. Mainquist for manufacturing the temperature device, A. Marelli and T. Orth for preparing miniprep DNA and M. Caterina for providing rat TRPV1 plasmid DNA. We thank T. Bartfai, E. Lattman, I. MacRae and A. Dubin for helpful discussion. This research was supported by grants from the US National Institutes of Health and by the Novartis Research Foundation.
Author information
Authors and Affiliations
Contributions
J.G. and H.H. contributed equally to this work. J.G. and H.H. designed the study, collected and analyzed data and wrote the manuscript. M.B. participated in designing the temperature device. B.B. conducted molecular modeling. M.S. carried out the biochemical experiments. M.P. participated in producing the random mutant library. A.P. designed the study and wrote the manuscript. All authors discussed results and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary Figures 1–3 and Supplementary Tables 1–4
Supplementary Text and Figures (PDF 408 kb)
Rights and permissions
About this article
Cite this article
Grandl, J., Hu, H., Bandell, M. et al. Pore region of TRPV3 ion channel is specifically required for heat activation. Nat Neurosci 11, 1007–1013 (2008). https://doi.org/10.1038/nn.2169
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2169
This article is cited by
-
Thermoring basis for the TRPV3 bio-thermometer
Scientific Reports (2023)
-
FRET analysis of the temperature-induced structural changes in human TRPV3
Scientific Reports (2023)
-
Structural mechanism of heat-induced opening of a temperature-sensitive TRP channel
Nature Structural & Molecular Biology (2021)
-
TRP Channels, Conformational Flexibility, and the Lipid Membrane
The Journal of Membrane Biology (2020)
-
STIM1 thermosensitivity defines the optimal preference temperature for warm sensation in mice
Cell Research (2019)