Abstract
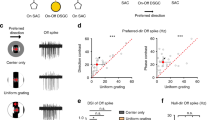
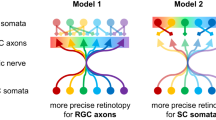
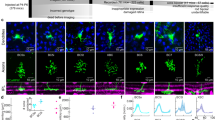
Sensory circuits frequently integrate converging inputs while maintaining precise functional relationships between them. For example, in mammals with stereopsis, neurons at the first stages of binocular visual processing show a close alignment of receptive-field properties for each eye. Still, basic questions about the global wiring mechanisms that enable this functional alignment remain unanswered, including whether the addition of a second retinal input to an otherwise monocular neural circuit is sufficient for the emergence of these binocular properties. We addressed this question by inducing a de novo binocular retinal projection to the larval zebrafish optic tectum and examining recipient neuronal populations using in vivo two-photon calcium imaging. Notably, neurons in rewired tecta were predominantly binocular and showed matching direction selectivity for each eye. We found that a model based on local inhibitory circuitry that computes direction selectivity using the topographic structure of both retinal inputs can account for the emergence of this binocular feature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hubel, D.H. & Wiesel, T.N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. (Lond.) 160, 106–154 (1962).
Maske, R., Yamane, S. & Bishop, P.O. Binocular simple cells for local stereopsis: comparison of receptive field organizations for the two eyes. Vision Res. 24, 1921–1929 (1984).
Sterling, P. & Wickelgren, B.G. Visual receptive fields in the superior colliculus of the cat. J. Neurophysiol. 32, 1–15 (1969).
Ohzawa, I., DeAngelis, G.C. & Freeman, R.D. Encoding of binocular disparity by simple cells in the cat's visual cortex. J. Neurophysiol. 75, 1779–1805 (1996).
Cynader, M. & Berman, N. Receptive-field organization of monkey superior colliculus. J. Neurophysiol. 35, 187–201 (1972).
Drager, U.C. & Hubel, D.H. Physiology of visual cells in mouse superior colliculus and correlation with somatosensory and auditory input. Nature 253, 203–204 (1975).
Stein, B.E., Magalhaes-Castro, B. & Kruger, L. Relationship between visual and tactile representations in cat superior colliculus. J. Neurophysiol. 39, 401–419 (1976).
Wallace, M.T., Meredith, M.A. & Stein, B.E. Integration of multiple sensory modalities in cat cortex. Exp. Brain Res. 91, 484–488 (1992).
Gödecke, I. & Bonhoeffer, T. Development of identical orientation maps for two eyes without common visual experience. Nature 379, 251–254 (1996).
Blakemore, C., Van Sluyters, R.C., Peck, C.K. & Hein, A. Development of cat visual cortex following rotation of one eye. Nature 257, 584–586 (1975).
Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Niell, C.M. & Smith, S.J. Functional imaging reveals rapid development of visual response properties in the zebrafish tectum. Neuron 45, 941–951 (2005).
Law, M.I. & Constantine-Paton, M. Right and left eye bands in frogs with unilateral tectal ablations. Proc. Natl. Acad. Sci. USA 77, 2314–2318 (1980).
Ruthazer, E.S., Akerman, C.J. & Cline, H.T. Control of axon branch dynamics by correlated activity in vivo. Science 301, 66–70 (2003).
Constantine-Paton, M. & Law, M.I. Eye-specific termination bands in tecta of three-eyed frogs. Science 202, 639–641 (1978).
Levine, R.L. & Jacobson, M. Discontinuous mapping of retina onto tectum innervated by both eyes. Brain Res. 98, 172–176 (1975).
Udin, S.B. & Grant, S. Plasticity in the tectum of Xenopus laevis: binocular maps. Prog. Neurobiol. 59, 81–106 (1999).
Crair, M.C., Gillespie, D.C. & Stryker, M.P. The role of visual experience in the development of columns in cat visual cortex. Science 279, 566–570 (1998).
Hubel, D.H. & Wiesel, T.N. Binocular interaction in striate cortex of kittens reared with artificial squint. J. Neurophysiol. 28, 1041–1059 (1965).
Katz, L.C. & Crowley, J.C. Development of cortical circuits: lessons from ocular dominance columns. Nat. Rev. Neurosci. 3, 34–42 (2002).
Wong, R.O., Meister, M. & Shatz, C.J. Transient period of correlated bursting activity during development of the mammalian retina. Neuron 11, 923–938 (1993).
Sharma, S.C. Anomalous retinal projection after removal of contralateral optic tectum in adult goldfish. Exp. Neurol. 41, 661–669 (1973).
Livingstone, M.S. & Conway, B.R. Substructure of direction-selective receptive fields in macaque V1. J. Neurophysiol. 89, 2743–2759 (2003).
Rao, R.P. & Sejnowski, T.J. Predictive learning of temporal sequences in recurrent neocortical circuits. Novartis Found. Symp. 239, 208–229 (2001).
Engert, F., Tao, H.W., Zhang, L.I. & Poo, M.M. Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature 419, 470–475 (2002).
Sajovic, P. & Levinthal, C. Inhibitory mechanism in zebrafish optic tectum: visual response properties of tectal cells altered by picrotoxin and bicuculline. Brain Res. 271, 227–240 (1983).
Higashijima, S., Mandel, G. & Fetcho, J.R. Distribution of prospective glutamatergic, glycinergic and GABAergic neurons in embryonic and larval zebrafish. J. Comp. Neurol. 480, 1–18 (2004).
Barlow, H.B. & Levick, W.R. The mechanism of directionally selective units in rabbit's retina. J. Physiol. (Lond.) 178, 477–504 (1965).
Wartzok, D. & Marks, W.B. Directionally selective visual units recorded in optic tectum of the goldfish. J. Neurophysiol. 36, 588–604 (1973).
Kim, I.J., Zhang, Y., Yamagata, M., Meister, M. & Sanes, J.R. Molecular identification of a retinal cell type that responds to upward motion. Nature 452, 478–482 (2008).
Newsome, W.T., Mikami, A. & Wurtz, R.H. Motion selectivity in macaque visual cortex. III. Psychophysics and physiology of apparent motion. J. Neurophysiol. 55, 1340–1351 (1986).
Emerson, R.C. & Gerstein, G.L. Simple striate neurons in the cat. II. Mechanisms underlying directional asymmetry and directional selectivity. J. Neurophysiol. 40, 136–155 (1977).
Stuermer, C.A., Rohrer, B. & Munz, H. Development of the retinotectal projection in zebrafish embryos under TTX-induced neural-impulse blockade. J. Neurosci. 10, 3615–3626 (1990).
Flanagan, J.G. Neural map specification by gradients. Curr. Opin. Neurobiol. 16, 59–66 (2006).
Livingstone, M.S. Mechanisms of direction selectivity in macaque V1. Neuron 20, 509–526 (1998).
Clifford, C.W. & Ibbotson, M.R. Fundamental mechanisms of visual motion detection: models, cells and functions. Prog. Neurobiol. 68, 409–437 (2002).
Burrill, J.D. & Easter, S.S. Jr. Development of the retinofugal projections in the embryonic and larval zebrafish (Brachydanio rerio). J. Comp. Neurol. 346, 583–600 (1994).
Roeser, T. & Baier, H. Visuomotor behaviors in larval zebrafish after GFP-guided laser ablation of the optic tectum. J. Neurosci. 23, 3726–3734 (2003).
White, L.E., Coppola, D.M. & Fitzpatrick, D. The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature 411, 1049–1052 (2001).
Knudsen, E.I. Dynamic space codes in the superior colliculus. Curr. Opin. Neurobiol. 1, 628–632 (1991).
Sperry, R.W. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl. Acad. Sci. USA 50, 703–710 (1963).
Lambot, M.A., Depasse, F., Noel, J.C. & Vanderhaeghen, P. Mapping labels in the human developing visual system and the evolution of binocular vision. J. Neurosci. 25, 7232–7237 (2005).
Knudsen, E.I., du Lac, S. & Esterly, S.D. Computational maps in the brain. Annu. Rev. Neurosci. 10, 41–65 (1987).
Williams, S.E. et al. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron 39, 919–935 (2003).
Lister, J.A., Robertson, C.P., Lepage, T., Johnson, S.L. & Raible, D.W. nacre encodes a zebrafish microphthalmia-related protein that regulates neural crest–derived pigment cell fate. Development 126, 3757–3767 (1999).
Fricke, C., Lee, J.S., Geiger-Rudolph, S., Bonhoeffer, F. & Chien, C.B. astray, a zebrafish roundabout homolog required for retinal axon guidance. Science 292, 507–510 (2001).
Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 10, 433–436 (1997).
Pelli, D.G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442 (1997).
Abramoff, M.D., Magelhaes, P.J. & Ram, S.J. Image processing with ImageJ. Biophotonics Int. 11, 36–42 (2004).
Drapeau, P., Ali, D.W., Buss, R.R. & Saint-Amant, L. In vivo recording from identifiable neurons of the locomotor network in the developing zebrafish. J. Neurosci. Methods 88, 1–13 (1999).
Acknowledgements
We extend our gratitude to A. Kampff for help in microscope construction, J. Bollmann for a suggestion on microscope optimization and M. Orger for insightful discussions. The authors thank M. Livingstone, M. Meister, T. Bonhoeffer, J. Lichtman, V. Murthy, A. Schier, B. Olvecsky and members of the Engert laboratory for comments on the manuscript and helpful discussions. This work was supported by a National Science Foundation Predoctoral Fellowship, a National Science and Engineering Graduate Fellowship (P.R.), a US National Institutes of Health grant (R01 EY014429-01A2) and funding from the McKnight and Dana Foundations (F.E.).
Author information
Authors and Affiliations
Contributions
P.R. carried out the experiments and analyzed the data. P.R. and F.E. designed the experiments and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 1696 kb)
Supplementary Video 1
Calcium imaging of binocular visually driven tectal population activity in a rewired larval zebrafish. Tectal neurons respond to visual stimulation with 3° moving spots shown to either the ipsilateral (left) or the contralateral (right) eye. Stimuli are shown schematically in the lower left corner. (AVI 32038 kb)
Rights and permissions
About this article
Cite this article
Ramdya, P., Engert, F. Emergence of binocular functional properties in a monocular neural circuit. Nat Neurosci 11, 1083–1090 (2008). https://doi.org/10.1038/nn.2166
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2166
This article is cited by
-
Evolution of central neural circuits: state of the art and perspectives
Nature Reviews Neuroscience (2022)
-
Focusing on optic tectum circuitry through the lens of genetics
BMC Biology (2010)
-
Monitoring neural activity with bioluminescence during natural behavior
Nature Neuroscience (2010)
-
In vivo single-cell excitability probing of neuronal ensembles in the intact and awake developing Xenopus brain
Nature Protocols (2010)
-
Constructing a road map from synapses to behaviour
EMBO reports (2009)