Abstract
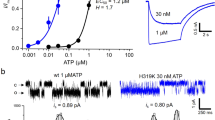
Three families of ligand-activated ion channels mediate synaptic communication between excitable cells in mammals. For pentameric channels related to nicotinic acetylcholine receptors and tetrameric channels such as glutamate receptors, the pore-forming and gate regions have been studied extensively. In contrast, little is known about the structure of trimeric P2X receptor channels, a family of channels that are activated by ATP and are important in neuronal signaling, pain transmission and inflammation. To identify the pore-forming and gate regions in P2X receptor channels, we introduced cysteine residues throughout the two transmembrane (TM) segments and studied their accessibility to thiol-reactive compounds and ions. Our results show that TM2 lines the central ion-conduction pore, TM1 is positioned peripheral to TM2 and the flow of ions is minimized in the closed state by a gate formed by the external region of TM2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Khakh, B.S. & North, R.A. P2X receptors as cell-surface ATP sensors in health and disease. Nature 442, 527–532 (2006).
North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 (2002).
Nicke, A. et al. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 17, 3016–3028 (1998).
Stoop, R. et al. Contribution of individual subunits to the multimeric P2X2 receptor: estimates based on methanethiosulfonate block at T336C. Mol. Pharmacol. 56, 973–981 (1999).
Jiang, L.H. et al. Subunit arrangement in P2X receptors. J. Neurosci. 23, 8903–8910 (2003).
Aschrafi, A., Sadtler, S., Niculescu, C., Rettinger, J. & Schmalzing, G. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J. Mol. Biol. 342, 333–343 (2004).
Barrera, N.P., Ormond, S.J., Henderson, R.M., Murrell-Lagnado, R.D. & Edwardson, J.M. Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers, but that P2X6 receptor subunits do not oligomerize. J. Biol. Chem. 280, 10759–10765 (2005).
Evans, R.J. Orthosteric and allosteric binding sites of P2X receptors. Eur. Biophys. J. published online, doi: 10.1007/s00249-008-0275-2 (5 February 2008).
Silberberg, S.D., Chang, T.H. & Swartz, K.J. Secondary structure and gating rearrangements of transmembrane segments in rat P2X4 receptor channels. J. Gen. Physiol. 125, 347–359 (2005).
Li, Z., Migita, K., Samways, D.S., Voigt, M.M. & Egan, T.M. Gain and loss of channel function by alanine substitutions in the transmembrane segments of the rat ATP-gated P2X2 receptor. J. Neurosci. 24, 7378–7386 (2004).
Holmgren, M., Liu, Y., Xu, Y. & Yellen, G. On the use of thiol-modifying agents to determine channel topology. Neuropharmacology 35, 797–804 (1996).
Liu, Y., Holmgren, M., Jurman, M.E. & Yellen, G. Gated access to the pore of a voltage-dependent K+ channel. Neuron 19, 175–184 (1997).
del Camino, D. & Yellen, G. Tight steric closure at the intracellular activation gate of a voltage- gated K+ channel. Neuron 32, 649–656 (2001).
Haines, W.R., Voigt, M.M., Migita, K., Torres, G.E. & Egan, T.M. On the contribution of the first transmembrane domain to whole-cell current through an ATP-gated ionotropic P2X receptor. J. Neurosci. 21, 5885–5892 (2001).
Egan, T.M., Haines, W.R. & Voigt, M.M. A domain contributing to the ion channel of ATP-gated P2X2 receptors identified by the substituted cysteine accessibility method. J. Neurosci. 18, 2350–2359 (1998).
Rassendren, F., Buell, G., Newbolt, A., North, R.A. & Surprenant, A. Identification of amino acid residues contributing to the pore of a P2X receptor. EMBO J. 16, 3446–3454 (1997).
Jiang, L.H., Rassendren, F., Spelta, V., Surprenant, A. & North, R.A. Amino acid residues involved in gating identified in the first membrane-spanning domain of the rat P2X2 receptor. J. Biol. Chem. 276, 14902–14908 (2001).
Ennion, S.J. & Evans, R.J. Conserved cysteine residues in the extracellular loop of the human P2X1 receptor form disulfide bonds and are involved in receptor trafficking to the cell surface. Mol. Pharmacol. 61, 303–311 (2002).
Clyne, J.D., Wang, L.F. & Hume, R.I. Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J. Neurosci. 22, 3873–3880 (2002).
Silberberg, S.D., Li, M. & Swartz, K.J. Ivermectin interaction with transmembrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron 54, 263–274 (2007).
Stauffer, D.A. & Karlin, A. Electrostatic potential of the acetylcholine binding sites in the nicotinic receptor probed by reactions of binding-site cysteines with charged methanethiosulfonates. Biochemistry 33, 6840–6849 (1994).
Armstrong, C.M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58, 413–437 (1971).
Armstrong, C.M. Ionic pores, gates and gating currents. Q. Rev. Biophys. 7, 179–210 (1974).
Choi, K.L., Mossman, C., Aube, J. & Yellen, G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron 10, 533–541 (1993).
Cao, L., Young, M.T., Broomhead, H.E., Fountain, S.J. & North, R.A. Thr339-to-serine substitution in rat P2X2 receptor second transmembrane domain causes constitutive opening and indicates a gating role for Lys308. J. Neurosci. 27, 12916–12923 (2007).
Flynn, G.E. & Zagotta, W.N. Conformational changes in S6 coupled to the opening of cyclic nucleotide–gated channels. Neuron 30, 689–698 (2001).
Contreras, J.E., Srikumar, D. & Holmgren, M. Gating at the selectivity filter in cyclic nucleotide–gated channels. Proc. Natl. Acad. Sci. USA 105, 3310–3314 (2008).
Lu, Q. & Miller, C. Silver as a probe of pore-forming residues in a potassium channel. Science 268, 304–307 (1995).
Jasti, J., Furukawa, H., Gonzales, E.B. & Gouaux, E. Structure of acid-sensing ion channel 1 at 1.9-Å resolution and low pH. Nature 449, 316–323 (2007).
Doyle, D.A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998).
Migita, K., Haines, W.R., Voigt, M.M. & Egan, T.M. Polar residues of the second transmembrane domain influence cation permeability of the ATP-gated P2X2 receptor. J. Biol. Chem. 276, 30934–30941 (2001).
Egan, T.M. & Khakh, B.S. Contribution of calcium ions to P2X channel responses. J. Neurosci. 24, 3413–3420 (2004).
Yellen, G. The voltage-gated potassium channels and their relatives. Nature 419, 35–42 (2002).
Zhou, Y., Morais-Cabral, J.H., Kaufman, A. & MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel–Fab complex at 2.0-Å resolution. Nature 414, 43–48 (2001).
Brake, A.J., Wagenbach, M.J. & Julius, D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 371, 519–523 (1994).
Hamill, O.P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 (1981).
Acknowledgements
We thank J. Contreras and M. Holmgren for extensive advice in using the thiol-reactive compounds described in this study. We also thank M. Holmgren, J. Mindell and members of the Swartz lab for helpful discussions, and the US National Institute of Neurological Disorders and Stroke DNA sequencing facility for DNA sequencing. This work was supported by the Intramural Research Program of the US National Institute of Neurological Disorders and Stroke.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 and Supplementary Table 1 (PDF 1058 kb)
Rights and permissions
About this article
Cite this article
Li, M., Chang, TH., Silberberg, S. et al. Gating the pore of P2X receptor channels. Nat Neurosci 11, 883–887 (2008). https://doi.org/10.1038/nn.2151
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2151
This article is cited by
-
Unravelling the intricate cooperativity of subunit gating in P2X2 ion channels
Scientific Reports (2020)
-
Insights into the channel gating of P2X receptors from structures, dynamics and small molecules
Acta Pharmacologica Sinica (2016)
-
Physical basis of apparent pore dilation of ATP-activated P2X receptor channels
Nature Neuroscience (2015)
-
Molecular mechanism of ATP binding and ion channel activation in P2X receptors
Nature (2012)