Abstract
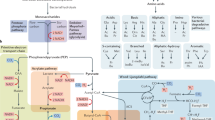
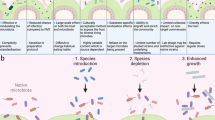
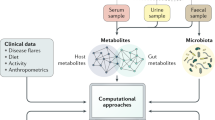
The increasing evidence pointing towards the involvement of the gut microbiome in multiple diseases, as well as its plasticity, renders it a desirable potential therapeutic target. Nevertheless, classical therapies based on the consumption of live probiotic bacteria, or their enrichment by prebiotics, exhibit limited efficacy. Recently, a novel therapeutic approach has been suggested based on metabolites secreted, modulated or degraded by the microbiome. As many of the host–microorganism interactions pertaining to human health are mediated by metabolites, this approach may be able to provide therapeutic efficacy while overcoming caveats of current microbiome-targeting therapies, such as colonization resistance and inter-individual variation in microbial composition. In this Perspective, we will discuss the evidence that supports pursuing the metabolite-based therapeutic approach as well as issues critical for its implementation. In a broader context, we will discuss how recent advances in microbiome research may improve and refine current treatment modalities, and the potential of combining them with metabolite-based interventions as a means of achieving a person-specific, integrated and efficient therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Collins, F. S., Morgan, M. & Patrinos, A. The Human Genome Project: lessons from large-scale biology. Science 300, 286–290 (2003).
Korem, T. et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 349, 1101–1106 (2015).
Fuller, R. A review. J. Appl. Bacteriol. 66, 365–378 (1989).
von Schillde, M.-A. et al. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe 11, 387–396 (2012).
Yan, F. et al. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J. Biol. Chem. 288, 30742–30751 (2013).
Probiotics: In Depth (NCCIH, 2016); https://nccih.nih.gov/health/probiotics/introduction.htm.
Zhu, A., Sunagawa, S., Mende, D. R. & Bork, P. Inter-individual differences in the gene content of human gut bacterial species. Genome Biol. 16, 82 (2015).
Nayfach, S., Rodriguez-Mueller, B., Garud, N. & Pollard, K. S. An integrated metagenomics pipeline for strain profiling reveals novel patterns of bacterial transmission and biogeography. Genome Res. 26, 1612–1625 (2016).
Maldonado-Gómez, M. X. et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe 20, 515–526 (2016).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 (2012).
Paramsothy, S. et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 389, 1218–1228 (2017).
Alang, N. & Kelly, C. R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2, ofv004 (2015).
Moayyedi, P. et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109 (2015).
Petrof, E. O. et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 1, 3 (2013).
Ott, S. J. et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile Infection. Gastroenterology (2016).
Glenn, G. & Roberfroid, M. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401–1412 (1995).
Bindels, L. B., Delzenne, N. M., Cani, P. D. & Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 12, 303–310 (2015).
Beserra, B. T. et al. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin. Nutr. 34, 845–858 (2015).
Ford, A. C. et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am. J. Gastroenterol. 109, 1547–1561 (2014).
Walker, A. W. et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 5, 220–230 (2011).
Kovatcheva-Datchary, P. et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 22, 971–982 (2015).
Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113 (2017).
van Nood, E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013).
Buffie, C. G. et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517, 205–208 (2015).
Levy, M. et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163, 1428–1443 (2015).
Patel, N. et al. Metabolomic analysis of breath volatile organic compounds reveals unique breathprints in children with inflammatory bowel disease: a pilot study. Ailment. Pharmacol. Ther. 40, 498–507 (2014).
De Preter, V. et al. Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut 64, 447–458 (2014).
Butzner, J., Parmar, R., Bell, C. & Dalal, V. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat. Gut 38, 568–573 (1996).
Scheppach, W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas a placebo-controlled trial. German-Austrian SCFA Study Group. Dig. Dis. Sci. 41, 2254–2259 (1996).
McIntyre, A., Gibson, P. & Young, G. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut 34, 386–391 (1993).
Hinnebusch, B. F., Meng, S., Wu, J. T., Archer, S. Y. & Hodin, R. A. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 132, 1012–1017 (2002).
De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96 (2014).
Gao, Z. et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517 (2009).
Lin, H. V. et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 7, e35240 (2012).
Donohoe, D. R. et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13, 517–526 (2011).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010).
Perry, R. J. et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534, 213–217 (2016).
Cassidy, A. & Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 105, 10–22 (2017).
Thaiss, C. A. et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 540, 544–551 (2016).
Thaiss, C. A. et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 167, 1495–1510 (2016).
Donia, M. S. & Fischbach, M. A. Small molecules from the human microbiota. Science 349, 1254766 (2015).
Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339–1353 (2016).
Suez, J. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186 (2014).
Klemashevich, C. et al. Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Curr. Opin. Biotechnol. 26, 85–90 (2014).
Acknowledgements
We thank the members of the Elinav Lab for fruitful discussions. We apologize to authors whose relevant work was not included in this Perspective owing to space constraints. J.S. is the recipient of the Strauss Institute research fellowship. E.E. is supported by Y. Ungar and R. Ungar, Israel; the Abisch Frenkel Foundation for the Promotion of Life Sciences; the Gurwin Family Fund for Scientific Research; the Leona M. and Harry B. Helmsley Charitable Trust; the Crown Endowment Fund for Immunological Research; the estate of J. Gitlitz; the estate of L. Hershkovich; the Benoziyo Endowment Fund for the Advancement of Science; the Adelis Foundation; J.L. Schwartz and V. Schwartz, Pacific Palisades; A. Markovitz, Canada; C. Adelson, Canada; CNRS (Centre National de la Recherche Scientifique); the estate of S. Weber and A.J. Weber; Mr and Mrs D.L. Schwarz, Sherman Oaks; grants funded by the European Research Council; the Kenneth Rainin Foundation; the German-Israel Binational Foundation; the Israel Science Foundation; the Minerva Foundation; the Rising Tide Foundation; and the Alon Foundation Scholar Award. E.E. is the incumbent of the Rina Gudinski Career Development Chair and is a senior fellow, CIFAR (Canadian Institute for Advanced Research).
Author information
Authors and Affiliations
Contributions
J.S. and E.E. chose the topics for the various sections, reviewed the literature, designed the figure and wrote the manuscript. Both authors contributed equally to the writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Suez, J., Elinav, E. The path towards microbiome-based metabolite treatment. Nat Microbiol 2, 17075 (2017). https://doi.org/10.1038/nmicrobiol.2017.75
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2017.75
This article is cited by
-
Triangulating nutrigenomics, metabolomics and microbiomics toward personalized nutrition and healthy living
Human Genomics (2023)
-
A probiotic bi-functional peptidoglycan hydrolase sheds NOD2 ligands to regulate gut homeostasis in female mice
Nature Communications (2023)
-
The Current and Future Perspectives of Postbiotics
Probiotics and Antimicrobial Proteins (2023)
-
AGAMEMNON: an Accurate metaGenomics And MEtatranscriptoMics quaNtificatiON analysis suite
Genome Biology (2022)
-
Plumping up a Cushion of Human Biowaste in Regenerative Medicine: Novel Insights into a State-of-the-Art Reserve Arsenal
Stem Cell Reviews and Reports (2022)