Abstract
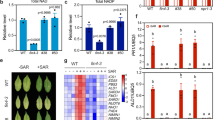
Understanding how microorganisms manipulate plant innate immunity and colonize host cells is a major goal of plant pathology. Here, we report that the fungal nitrooxidative stress response suppresses host defences to facilitate the growth and development of the important rice pathogen Magnaporthe oryzae in leaf cells. Nitronate monooxygenases encoded by NMO genes catalyse the oxidative denitrification of nitroalkanes. We show that the M. oryzae NMO2 gene is required for mitigating damaging lipid nitration under nitrooxidative stress conditions and, consequently, for using nitrate and nitrite as nitrogen sources. On plants, the Δnmo2 mutant strain penetrated host cuticles like wild type, but invasive hyphal growth in rice cells was restricted and elicited plant immune responses that included the formation of cellular deposits and a host reactive oxygen species burst. Development of the M. oryzae effector-secreting biotrophic interfacial complex (BIC) was misregulated in the Δnmo2 mutant. Inhibiting or quenching host reactive oxygen species suppressed rice innate immune responses and allowed the Δnmo2 mutant to grow and develop normally in infected cells. NMO2 is thus essential for mitigating nitrooxidative cellular damage and, in rice cells, maintaining redox balance to avoid triggering plant defences that impact M. oryzae growth and BIC development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wilson, R. A. & Talbot, N. J. Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 7, 185–195 (2009).
Fernandez, J. & Wilson, R. A. Why no feeding frenzy? Mechanisms of nutrient acquisition and utilization during infection by the rice blast fungus Magnaporthe oryzae. Mol. Plant Microbe Interact. 25, 1286–1293 (2012).
Martin-Urdiroz, M., Oses-Ruiz, M., Ryder, L. S. & Talbot, N. J. Investigating the biology of plant infection by the rice blast fungus Magnaporthe oryzae. Fungal Genet. Biol. 90, 61–68 (2016).
Ryder, L. S. & Talbot, N. J. Regulation of appressorium development in pathogenic fungi. Curr. Opin. Plant Biol. 26, 8–13 (2015).
Marroquin-Guzman, M. & Wilson, R. A. GATA-dependent glutaminolysis drives appressorium formation in Magnaporthe oryzae by suppressing TOR inhibition of cAMP/PKA signaling. PLoS Pathog. 11, e1004851 (2015).
Dagdas, Y. F. et al. Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science 336, 1590–1595 (2012).
Kankanala, P., Czymmek, K. & Valent, B. Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell 19, 706–724 (2007).
Fernandez, J. & Wilson, R. A. Cells in cells: morphogenetic and metabolic strategies conditioning rice infection by the blast fungus Magnaporthe oryzae. Protoplasma 251, 37–47 (2014).
Park, C.-H. et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell 24, 4748–4762 (2012).
Mentlak, T. A. et al. Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell 24, 322–335 (2012).
Giraldo, M. C. et al. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat. Commun. 4, 1996 (2013).
Fernandez, J., Marroquin-Guzman, M. & Wilson, R. A. Mechanisms of nutrient acquisition and utilization during fungal infections of leaves. Ann. Rev. Phytopathol. 52, 155–174 (2014).
Chi, M. H., Park, S. Y., Kim, S. & Lee, Y. H. A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host. PLoS Pathog. 5, e1000401 (2009).
Huang, K., Czymmek, K. J., Caplan, J. L., Sweigard, J. A. & Donofrio, N. M. HYR1-mediated detoxification of reactive oxygen species is required for full virulence in the rice blast fungus. PLoS Pathog. 7, e1001335 (2011).
Donofrio, N. M. & Wilson, R. A. Redox and rice blast: new tools for dissecting molecular fungal–plant interactions. New Phytol. 201, 367–369 (2014).
Fernandez, J. et al. Plant defense suppression is mediated by a fungal sirtuin during rice infection by Magnaporthe oryzae. Mol. Micro. 94, 70–88 (2014).
Fernandez, J. et al. Principles of carbon catabolite repression in the rice blast fungus: Tps1, Nmr1-3, and a MATE-family pump regulate glucose metabolism during infection. PLoS Genet. 8, e1002673 (2012).
Fernandez, J. & Wilson, R. A. Characterizing roles for the glutathione reductase, thioredoxin reductase and thioredoxin peroxidase-encoding genes of Magnaporthe oryzae during rice blast disease. PLoS ONE 9, e87300 (2014).
Dean, R. A. et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434, 980–986 (2005).
Wilson, R. A. & Arst, H. N. Jr. Mutational analysis of AREA, a transcriptional activator mediating nitrogen metabolite repression in Aspergillus nidulans and a member of the ‘streetwise’ GATA family of transcription factors. Microbiol. Mol. Biol. Rev. 62, 586–596 (1998).
Wilson, R. A., Gibson, R. P., Quispe, C. F., Littlechild, J. A. & Talbot, N. J. An NADPH-dependent genetic switch regulates plant infection by the rice blast fungus. Proc. Natl Acad. Sci. USA 107, 21902–21907 (2010).
Wilson, R. A. et al. Tps1 regulates the pentose phosphate pathway, nitrogen metabolism and fungal virulence. EMBO J. 26, 3673–3685 (2007).
Marcos, A. T. et al. Nitric oxide synthesis by nitrate reductase is regulated during development in Aspergillus. Mol. Microbiol. 99, 15–33 (2016).
Corpas, F. J. & Barroso, J. B. Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol. 199, 633–635 (2013).
O'Donnell, V. B. & Freeman, B. A. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ. Res. 88, 12–21 (2001).
Pacher, P., Beckman, J. S. & Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87, 315–424 (2007).
Vandelle, E. & Delledonne, M. Peroxynitrite formation and function in plants. Plant Sci. 181, 534–539 (2011).
Nathan, C. The moving frontier in nitric oxide-dependent signaling. Sci. STKE 2004, pe52 (2004).
Rubbo, H. & Radi, R. Protein and lipid nitration: role in redox signaling and injury. Biochim. Biophys. Acta 1780, 1318–1324 (2008).
Batinić-Haberle, I. et al. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two different models of oxidative stress injuries, SOD-specific E. coli model and carrageenan-induced pleurisy. Free Radic. Biol. Med. 46, 192–201 (2009).
Radi, R., Beckman, J. S., Bush, K. M. & Freeman, B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 288, 481–487 (1991).
Ischiropoulos, H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch. Biochem. Biophys. 356, 1–11 (1998).
Hao, P. et al. Herbivore-induced callose depositions on the sieve plates of rice: an important mechanism for host resistance. Plant Physiol. 146, 1810–1820 (2008).
Khang, C. H. et al. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 22, 1388–13403 (2010).
Brown, A. J., Haynes, K. & Quinn, J. Nitrosative and oxidative stress responses in fungal pathogenicity. Curr. Opin. Microbiol. 12, 384–391 (2009).
Francis, K., Russell, B. & Gadda, G. Involvement of a flavosemiquinone in the enzymatic oxidation of nitroalkanes catalyzed by 2-nitropropane dioxygenase. J. Biol. Chem. 280, 5195–51204 (2005).
Bellin, D., Asai, S., Delledonne, M. & Yoshioka, H. Nitric oxide as a mediator for defense responses. Mol. Plant Microbe Interact. 26, 271–277 (2013).
Li, G., Marroquin-Guzman, M. & Wilson, R. A. Chromatin immunoprecipitation (ChIP) assay for detecting direct and indirect protein–DNA interactions in Magnaporthe oryzae. Bio Protoc 5, e1643 (2015).
Kido, T., Soda, K., Suzuki, T. & Asada, K. A new oxygenase, 2-nitropropane dioxygenase of Hansenula mrakii. Enzymologic and spectrophotometric properties. J. Biol. Chem. 251, 6994–7000 (1976).
Grossi, L. & D'Angelo, S. Sodium nitroprusside: mechanism of NO release mediated by sulfhydryl-containing molecules. J. Med. Chem. 48, 2622–2626 (2005).
Acknowledgements
The authors thank B. Valent (Kansas State University) for the gift of pBV591. This work was supported by grants from the National Science Foundation (IOS-1557943) and the USDA-NIFA (2014-67013-21559) to R.A.W. A UNL ARD bridging fund award supported M.M.-G.
Author information
Authors and Affiliations
Contributions
R.A.W. conceived the project, designed the experiments and interpreted the data. M.M.-G., D.H., J.D.W., C.E., T.J.B. and R.A.W. performed experiments and analysed the data. R.A.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–3; Supplementary Tables 1–2 (PDF 574 kb)
Rights and permissions
About this article
Cite this article
Marroquin-Guzman, M., Hartline, D., Wright, J. et al. The Magnaporthe oryzae nitrooxidative stress response suppresses rice innate immunity during blast disease. Nat Microbiol 2, 17054 (2017). https://doi.org/10.1038/nmicrobiol.2017.54
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2017.54
This article is cited by
-
Enhanced ultraviolet-B radiation alleviates structural damages on rice leaf caused by Magnaporthe oryzae infection
Protoplasma (2024)
-
A protein kinase coordinates cycles of autophagy and glutaminolysis in invasive hyphae of the fungus Magnaporthe oryzae within rice cells
Nature Communications (2023)
-
Unconventional secretion of Magnaporthe oryzae effectors in rice cells is regulated by tRNA modification and codon usage control
Nature Microbiology (2023)
-
Revisiting the Critical Role of ROS and RNS in Plant Defense
Journal of Plant Growth Regulation (2023)
-
Glycine-derived nitronates bifurcate to O-methylation or denitrification in bacteria
Nature Chemistry (2021)