Abstract
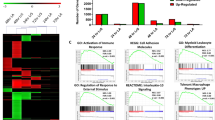
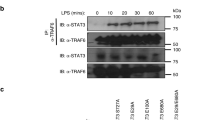
Microbial stimuli such as lipopolysaccharide (LPS) induce robust metabolic rewiring in immune cells known as the Warburg effect. It is unknown whether this increase in glycolysis and decrease in oxidative phosphorylation (OXPHOS) is a general characteristic of monocytes that have encountered a pathogen. Using CD14+ monocytes from healthy donors, we demonstrated that most microbial stimuli increased glycolysis, but that only stimulation of Toll-like receptor (TLR) 4 with LPS led to a decrease in OXPHOS. Instead, activation of other TLRs, such as TLR2 activation by Pam3CysSK4 (P3C), increased oxygen consumption and mitochondrial enzyme activity. Transcriptome and metabolome analysis of monocytes stimulated with P3C versus LPS confirmed the divergent metabolic responses between both stimuli, and revealed significant differences in the tricarboxylic acid cycle, OXPHOS and lipid metabolism pathways following stimulation of monocytes with P3C versus LPS. At a functional level, pharmacological inhibition of complex I of the mitochondrial electron transport chain diminished cytokine production and phagocytosis in P3C- but not LPS-stimulated monocytes. Thus, unlike LPS, complex microbial stimuli and the TLR2 ligand P3C induce a specific pattern of metabolic rewiring that involves upregulation of both glycolysis and OXPHOS, which enables activation of host defence mechanisms such as cytokine production and phagocytosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
14 July 2017
In the PDF version of this article previously published, the year of publication provided in the footer of each page and in the 'How to cite' section was erroneously given as 2017, it should have been 2016. This error has now been corrected. The HTML version of the article was not affected.
References
Warburg, O., Wind, F. & Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530 (1927).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Rodriguez-Prados, J. C. et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 185, 605–614 (2010).
Galván-Peña, S. & O'Neill, L. A. Metabolic reprograming in macrophage polarization. Front. Immunol. 5, 420 (2014).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Jha, A. K. et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430 (2015).
Mills, E. & O'Neill, L. A. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 24, 313–320 (2014).
Everts, B. et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat. Immunol. 15, 323–332 (2014).
Tan, Z. et al. The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J. Biol. Chem. 290, 46–55 (2015).
Izquierdo, E. et al. Reshaping of human macrophage polarization through modulation of glucose catabolic pathways. J. Immunol. 195, 2442–2451 (2015).
Huang, S. C. et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 15, 846–855 (2014).
Palsson-McDermott, E. M. et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab. 21, 65–80 (2015).
Picard, M., Shirihai, O. S., Gentil, B. J. & Burelle, Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R393–406 (2013).
Chacko, B. K. et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Invest. 93, 690–700 (2013).
Krawczyk, C. M. et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115, 4742–4749 (2010).
Kelly, B., Tannahill, G. M., Murphy, M. P. & O'Neill, L. A. Metformin inhibits the production of reactive oxygen species from nADH:ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J. Biol. Chem. 290, 20348–20359 (2015).
O'Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Strelko, C. L. et al. Itaconic acid is a mammalian metabolite induced during macrophage activation. J. Am. Chem. Soc. 133, 16386–16389 (2011).
Takeuchi, O. et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11, 443–451 (1999).
Chandak, P. G. et al. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 285, 20192–20201 (2010).
Cifarelli, A., Pepe, G., Paradisi, F. & Piccolo, D. The influence of some metabolic inhibitors on phagocytic activity of mouse macrophages in vitro. Res. Exp. Med. 174, 197–204 (1979).
Paradisi, F., D'Onofrio, C., Pepe, G., Cifarelli, A. & Piccolo, D. Phagocytosis and cellular metabolism. Ric. Clin. Lab. 9, 47–60 (1979).
Jiang, Z., Mak, T. W., Sen, G. & Li, X. Toll-like receptor 3-mediated activation of NF-κB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-β. Proc. Natl Acad. Sci. USA 101, 3533–3538 (2004).
Odegaard, J. I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Majai, G., Sarang, Z., Csomos, K., Zahuczky, G. & Fesus, L. PPARγ-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur. J. Immunol. 37, 1343–1354 (2007).
Buck, M. D. et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 166, 63–76 (2016).
Kelly, B. & O'Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784 (2015).
Lampropoulou, V. et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metabol. 24, 158–166 (2016).
Li, Y. et al. Immune responsive gene 1 (IRG1) promotes endotoxin tolerance by increasing A20 expression in macrophages through reactive oxygen species. J. Biol. Chem. 288, 16225–16234 (2013).
Cheng, S. C. et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat. Immunol. 17, 406–413 (2016).
Janssen, A. J. et al. Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin. Chem. 53, 729–734 (2007).
Mourmans, J. et al. Clinical heterogeneity in respiratory chain complex III deficiency in childhood. J. Neurol. Sci. 149, 111–117 (1997).
Cooperstein, S. J. & Lazarow, A. A microspectrophotometric method for the determination of cytochrome oxidase. J. Biol. Chem. 189, 665–670 (1951).
Rodenburg, R. J. Biochemical diagnosis of mitochondrial disorders. J. Inherit. Metab. Dis. 34, 283–292 (2011).
Xia, J., Sinelnikov, I. V., Han, B. & Wishart, D. S. Metaboanalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 43, W251–257 (2015).
Bolstad, B. M., Irizarry, R. A., Astrand, M. & Speed, T. P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 (2003).
Irizarry, R. A. et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15 (2003).
Dai, M. et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 33, e175 (2005).
Sartor, M. A. et al. Intensity-based hierarchical Bayes method improves testing for differentially expressed genes in microarray experiments. BMC Bioinformatics 7, 538 (2006).
Blankley, S. et al. Identification of the key differential transcriptional responses of human whole blood following TLR2 or TLR4 ligation in-vitro. PLoS ONE 9, e97702 (2014).
Ramilo, O. et al. Gene expression patterns in blood leukocytes discriminate patients with acute infections. Blood 109, 2066–2077 (2007).
Acknowledgements
We would like to thank the laboratory technicians of the muscle laboratory, and in particular B. Stoltenborg, at the Translational Metabolic Laboratory (Department of Laboratory Medicine, Radboud University Nijmegen Medical Centre) and Mietske Wijers-Rouw (Department of Cell Biology, Radboud University Nijmegen Medical Centre) for excellent technical assistance. R.S. was supported by a VIDI grant from the The Netherlands Organisation for Scientific Research (NWO) and an EFSD Rising Star Grant. R.v.C. was supported by The European Union's Seventh Framework Programme (EU FP7) project TANDEM (HEALTH-F3-2012-305279). M.G.N. was supported by an ERC Consolidator Grant (no. 310372) and a Spinoza Award (NWO).
Author information
Authors and Affiliations
Contributions
E.L., L.B., J.M.R., A.H. and R.S. conducted most of the experiments and data analysis. G.J.H. performed all data analysis related to the transcriptome and metabolome results. R.J.R. and R.H.H. assisted in the experiments related to assessing mitochondrial function. J.A.M.F. performed the electron microscopy. L.A.B.J., R.H.H., R.v.C. and M.G.N. critically contributed to the design of the study. E.L., L.B., J.M.R., M.G.N. and R.S. wrote the manuscript together.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary Figures 1–8; Supplementary Table 1 (PDF 859 kb)
Rights and permissions
About this article
Cite this article
Lachmandas, E., Boutens, L., Ratter, J. et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat Microbiol 2, 16246 (2017). https://doi.org/10.1038/nmicrobiol.2016.246
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2016.246
This article is cited by
-
A Lacticaseibacillus rhamnosus secretome induces immunoregulatory transcriptional, functional and immunometabolic signatures in human THP-1 monocytes
Scientific Reports (2024)
-
NAD(H) homeostasis underlies host protection mediated by glycolytic myeloid cells in tuberculosis
Nature Communications (2023)
-
mTOR regulation of metabolism limits LPS-induced monocyte inflammatory and procoagulant responses
Communications Biology (2022)
-
Transcriptional reprogramming from innate immune functions to a pro-thrombotic signature by monocytes in COVID-19
Nature Communications (2022)
-
Immunometabolic crosstalk during bacterial infection
Nature Microbiology (2022)