Abstract
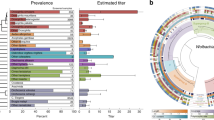
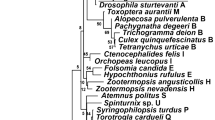
The genus Wolbachia (Alphaproteobacteria) comprises the most abundant inherited intracellular bacteria1. Despite their relevance as manipulators of human pathogen transmission2 and arthropod reproduction3, many aspects of their evolutionary history are not well understood4. In arthropods, Wolbachia infections are typically transient on evolutionary timescales5,6 and co-divergence between hosts and Wolbachia is supposedly rare. Consequently, much of our knowledge of Wolbachia genome evolution derives from very recently diverged strains, and a timescale for Wolbachia is lacking. Here, we investigated the genomes of four Wolbachia strains that have persisted within and co-diverged with their host lineage for ∼2 million years. Although the genomes showed very little evolutionary change on a nucleotide level, we found evidence for a recent lateral transfer of a complete biotin synthesis operon that has the potential to transform Wolbachia–host relationships7. Furthermore, this evolutionary snapshot enabled us to calibrate the divergence times of the supergroup A and B Wolbachia lineages using genome-wide data sets and relaxed molecular clock models. We estimated the origin of Wolbachia supergroups A and B to be ∼200 million years ago (Ma), which is considerably older than previously appreciated. This age coincides with the diversification of many insect lineages8 that represent most of Wolbachia’s host spectrum.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
14 July 2017
In the PDF version of this article previously published, the year of publication provided in the footer of each page and in the 'How to cite' section was erroneously given as 2017, it should have been 2016. This error has now been corrected. The HTML version of the article was not affected.
References
Weinert, L. A., Araujo-Jnr, E. V., Ahmed, M. Z. & Welch, J. J. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. Lond. B 282, 20150249 (2015).
Walker, T. et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476, 450–453 (2011).
Werren, J. H., Baldo, L. & Clark, M. E. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751 (2008).
Gerth, M., Gansauge, M.-T., Weigert, A. & Bleidorn, C. Phylogenomic analyses uncover origin and spread of the Wolbachia pandemic. Nat. Commun. 5, 5117 (2014).
Gerth, M., Röthe, J. & Bleidorn, C. Tracing horizontal Wolbachia movements among bees (Anthophila): a combined approach using MLST data and host phylogeny. Mol. Ecol. 22, 6149–6162 (2013).
Raychoudhury, R., Baldo, L., Oliveira, D. C. S. G. & Werren, J. H. Modes of acquisition of Wolbachia: horizontal transfer, hybrid introgression, and codivergence in the Nasonia species complex. Evolution 63, 165–183 (2009).
Nikoh, N. et al. Evolutionary origin of insect–Wolbachia nutritional mutualism. Proc. Natl Acad. Sci. USA 111, 10257–10262 (2014).
Misof, B. et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767 (2014).
Glowska, E., Dragun-Damian, A., Dabert, M. & Gerth, M. New Wolbachia supergroups detected in quill mites (Acari: Syringophilidae). Infect. Genet. Evol. 30, 140–146 (2015).
Zug, R. & Hammerstein, P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 7, e38544 (2012).
Teixeira, L., Ferreira, A., Ashburner, M. & Keller L. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6, e1000002 (2008).
Zabalou, S. et al. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl Acad. Sci. USA 101, 15042–15045 (2004).
Toomey, M. E., Panaram, K., Fast, E. M., Beatty, C. & Frydman, H. M. Evolutionarily conserved Wolbachia-encoded factors control pattern of stem-cell niche tropism in Drosophila ovaries and favor infection. Proc. Natl Acad. Sci. USA 110, 10788–10793 (2013).
Baldo, L. et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 72, 7098–7110 (2006).
Ellegaard, K. M., Klasson, L., Näslund, K., Bourtzis, K. & Andersson, S. G. E. Comparative genomics of Wolbachia and the bacterial species concept. PLoS Genet. 9, e1003381 (2013).
Choi, J. Y., Bubnell, J. E. & Aquadro, C. F. Population genomics of infectious and integrated Wolbachia pipientis genomes in Drosophila ananassae. Genome Biol. Evol. 7, 2362–2382 (2015).
Penz, T. et al. Comparative genomics suggests an independent origin of cytoplasmic incompatibility in Cardinium hertigii. PLoS Genet. 8, e1003012 (2012).
Linskens, H. F. & Stanley, R. G. Pollen: Biology, Biochemistry, Management (Springer, 1974).
Ochman, H. & Wilson, A. C. Evolution in bacteria: evidence for a universal substitution rate in cellular genomes. J. Mol. Evol. 26, 74–86 (1987).
Kuo, C.-H. & Ochman, H. Inferring clocks when lacking rocks: the variable rates of molecular evolution in bacteria. Biol. Direct 4, 35 (2009).
Chen, J.-Q. et al. Variation in the ratio of nucleotide substitution and indel rates across genomes in mammals and bacteria. Mol. Biol. Evol. 26, 1523–1531 (2009).
Moran, N. A., Munson, M. A., Baumann, P. & Ishikawa, H. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc. R. Soc. Lond. B 253, 167–171 (1993).
Bandi, C., Anderson, T. J. C., Genchi, C. & Blaxter, M. L. Phylogeny of Wolbachia in filarial nematodes. Proc. R. Soc. Lond. B 265, 2407–2413 (1998).
Werren, J. H., Zhang, W. & Guo, L. R. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. R. Soc. Lond. B 261, 55–63 (1995).
De Vienne, D. M. et al. Cospeciation vs host-shift speciation: methods for testing, evidence from natural associations and relation to coevolution. New Phytol. 198, 347–385 (2013).
Atyame, C. M., Delsuc, F., Pasteur, N., Weill, M. & Duron, O. Diversification of Wolbachia endosymbiont in the Culex pipiens mosquito. Mol. Biol. Evol. 28, 2761–2772 (2011).
Jaenike, J. & Dyer, K. A. No resistance to male-killing Wolbachia after thousands of years of infection. J. Evol. Biol. 21, 1570–1577 (2008).
Newton, I. L. G. et al. Comparative genomics of two closely related Wolbachia with different reproductive effects on hosts. Genome Biol. Evol. 8, 1526–1542 (2016).
Benton, M., Donoghue, P. C. J. & Asher, R. J. in The Timetree of Life (eds Hedges, S. B. & Kumar, S. ) 35–86 (Oxford Univ. Press, 2009).
Rehm, P. et al. Dating the arthropod tree based on large-scale transcriptome data. Mol. Phylogenet. Evol. 61, 880–887 (2011).
Cardinal, S. & Danforth, B. N. Bees diversified in the age of eudicots. Proc. R. Soc. Lond. B 280, 20122686 (2013).
Kircher, M., Sawyer, S. & Meyer, M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 40, e3 (2012).
Meyer, M. & Kircher, M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb. Protoc. http://dx.doi.org/10.1101/pdb.prot5448 (2010).
Renaud, G., Kircher, M., Stenzel, U. & Kelso, J. Freeibis: an efficient basecaller with calibrated quality scores for Illumina sequencers. Bioinformatics 29, 1208–1209 (2013).
Peng, Y. et al. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 28, 1420–1428 (2012).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinformatics 10, 421 (2009).
Sedlazeck, F. J., Rescheneder, P. & von Haeseler, A. Nextgenmap: fast and accurate read mapping in highly polymorphic genomes. Bioinformatics 29, 2790–2791 (2013).
Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2016).
Aziz, R. K. et al. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9, 75 (2008).
Emms, D. M. & Kelly, S. Orthofinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 16, 157 (2015).
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004).
Siguier, P., Perochon, J., Lestrade, L., Mahillon, J. & Chandler, M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34, D32–D36 (2006).
Darling, A. E., Mau, B. & Perna, N. T. ProgressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 5, e11147 (2010).
Guy, L., Roat Kultima, J. & Andersson, S. G. E. Genoplotr: comparative gene and genome visualization in R. Bioinformatics 26, 2334–2335 (2010).
Hartig, G. et al. Oligonucleotide primers for targeted amplification of single-copy nuclear genes in apocritan Hymenoptera. PLoS ONE 7, e39826 (2012).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Liu, L., Yu, L. & Edwards, S. V. A maximum pseudo-likelihood approach for estimating species trees under the coalescent model. BMC Evol. Biol. 10, 302 (2010).
Liu, L. & Yu, L. Estimating species trees from unrooted gene trees. Syst. Biol. 60, 661–667 (2011).
Shaw, T. I., Ruan, Z., Glenn, T. C. & Liu, L. STRAW: Species TRee analysis Web server. Nucleic Acids Res. 41, W238–W241 (2013).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Larsson, A. Aliview: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30, 3276–3278 (2014).
Xia, X., Xie, Z., Salemi, M., Chen, L. & Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 26, 1–7 (2003).
Xia, X. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 30, 1720–1728 (2013).
Bruen, T. C., Philippe, H. & Bryant, D. A simple and robust statistical test for detecting the presence of recombination. Genetics 172, 2665–2681 (2006).
Lanfear, R., Calcott, B., Ho, S. Y. W. & Guindon, S. Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701 (2012).
Minh, B. Q., Nguyen, M. A. T. & von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 30, 1188–1195 (2013).
Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003).
Lartillot, N., Rodrigue, N., Stubbs, D. & Richer, J. Phylobayes MPI: phylogenetic reconstruction with infinite mixtures of profiles in a parallel environment. Syst. Biol. 62, 611–615 (2013).
Lartillot, N. & Philippe, H. A Bayesian mixture model for across-site heterogeneities in the amino-acid replacement process. Mol. Biol. Evol. 21, 1095–1109 (2004).
Charif, D. & Lobry, J. in Structural Approaches to Sequence Evolution (eds. Bastolla, U., Porto, M., Roman, H. E. & Vendruscolo, M. ) 207–232 (Springer, 2007).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Duchêne, S., Molak, M. & Ho, S. Y. W. Clockstar: choosing the number of relaxed-clock models in molecular phylogenetic analysis. Bioinformatics 30, 1017–1019 (2013).
Rambaut, A., Suchard, M. A., Xie, D. & Drummond, A. J. Tracer v.1.6 (BEAST, 2014); http://beast.bio.ed.ac.uk/Tracer
Acknowledgements
We thank R. Sontowski and A. Weigert for laboratory assistance. M.G. is funded by the European Molecular Biology Organization (ALTF 48-2015) and co-funded by Marie-Curie Actions of the European Commission (LTFCOFUND2013, GA-2013-609409). C.B. is a ‘Ramon y Cajal’ fellow supported by the Spanish Ministry of Science and Education (MEC) (RYC-2014-15615). This work was supported by the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig.
Author information
Authors and Affiliations
Contributions
M.G. and C.B. conceived and designed the study, and wrote the paper. M.G. performed the in silico and in vitro procedures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-7, Supplementary Tables 1-4, Supplementary References. (PDF 1447 kb)
Rights and permissions
About this article
Cite this article
Gerth, M., Bleidorn, C. Comparative genomics provides a timeframe for Wolbachia evolution and exposes a recent biotin synthesis operon transfer. Nat Microbiol 2, 16241 (2017). https://doi.org/10.1038/nmicrobiol.2016.241
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2016.241
This article is cited by
-
Supergroup F Wolbachia with extremely reduced genome: transition to obligate insect symbionts
Microbiome (2023)
-
Effect of high temperature on Wolbachia density and impact on cytoplasmic incompatibility in confused flour beetle, Tribolium confusum (Coleoptera: Tenebrionidae)
BMC Research Notes (2022)
-
Complete de novo assembly of Wolbachia endosymbiont of Diaphorina citri Kuwayama (Hemiptera: Liviidae) using long-read genome sequencing
Scientific Reports (2022)
-
Multiple long-range host shifts of major Wolbachia supergroups infecting arthropods
Scientific Reports (2022)
-
The diversity of Wolbachia and its effects on host reproduction in a single Adalia bipunctata (Coleoptera: Coccinellidae) population
Symbiosis (2021)